Culture method for in-vitro-amplified-gene edited and activated T cells, kit and application
A gene editing and in vitro amplification technology, applied in the field of biotechnology and cell culture, can solve the problems of inability to meet scientific research needs, low efficiency, relying on natural recombination, etc., and achieve the effect of fast and efficient induction method, economical simplicity, and stable effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
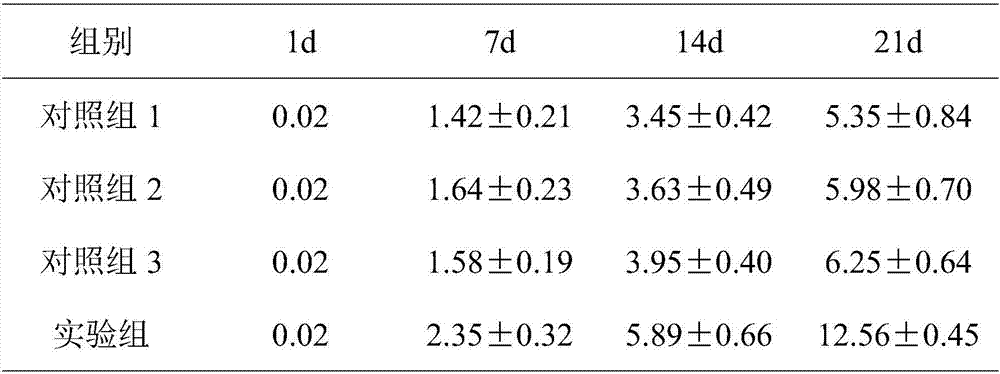
[0024] Embodiment 1 (experimental group)
[0025] Isolation and efficient expansion of human PD-1 gene knockout T cells using CRISPR-Cas9 technology
[0026] Human peripheral blood is now used as the processing sample, and the human PD-1 gene is knocked out by using CRISPR-Cas9 technology to construct PD-1 knockout T cells, and the gene-edited activated T cells are efficiently expanded and cultured in vitro. Specific steps are as follows:
[0027] Step 1: Isolation of peripheral blood mononuclear cells from blood
[0028] Directly extract 80-100ml of venous blood from the donor, and add anticoagulant-heparin;
[0029] Centrifuge the collected blood sample at 2000rpm for 10min at room temperature, and carefully absorb the upper plasma layer for later use; restore the blood sample to its original volume and mix well; slowly add the diluted blood to Ficoll, and centrifuge at 1000rpm for 20min; absorb milky white mononuclear cells at the interface of the separation solution lay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com