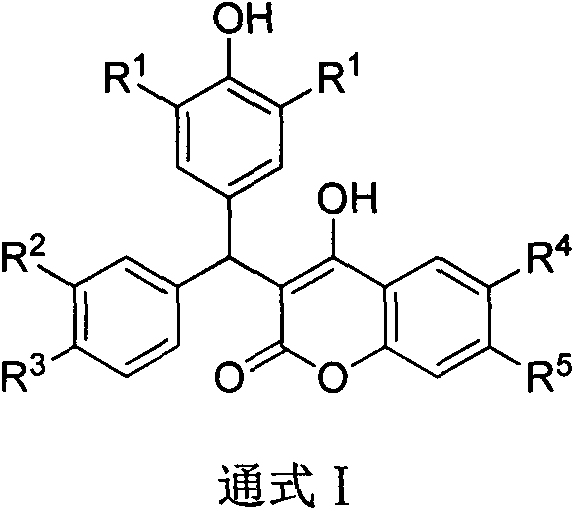
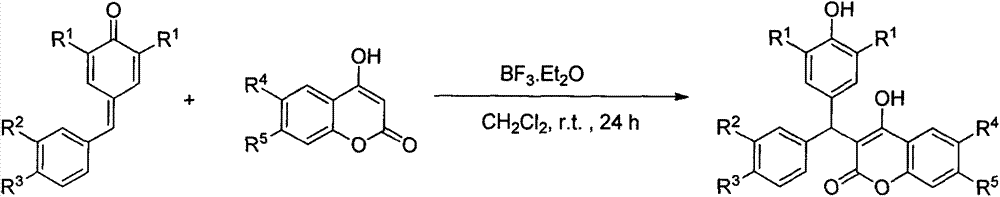
3-diaryl methane-4-hydroxycoumarin derivative preparation method
A technology of hydroxycoumarin and diarylmethane, which is applied in the field of preparation of 3-diarylmethane-4-hydroxycoumarin derivatives, can solve unfavorable environmental protection and industrialized production, poor regioselectivity, and reaction conditions harsh problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
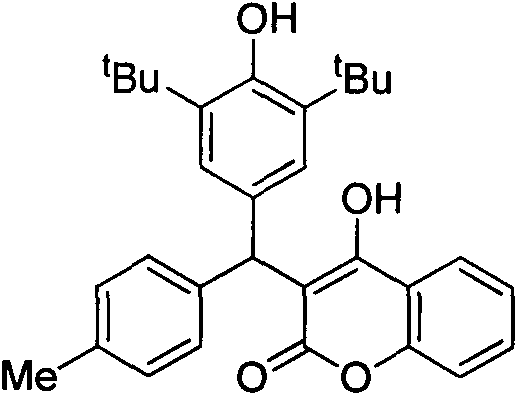
[0016] 3-((3,5-di-tert-butyl-4-hydroxyphenyl)(4-methylphenyl)methyl)-4-hydroxycoumarin (1)
[0017]
[0018] Take a reaction tube, add 2,6-di-tert-butyl-4-(4-methylbenzylidene)cyclohexa-2,5-dienone (61mg, 0.2mmol, 1.0equiv), 4-hydroxy Coumarin (38mg, 0.24mmol, 1.2equiv), boron trifluoride diethyl ether (0.04mmol, 0.2equiv), 1mL of dichloromethane, reacted at room temperature for 24 hours, evaporated the solvent under reduced pressure and separated through a silica gel column to obtain White solid product 84mg, yield 90%.
[0019] 1 H NMR (300MHz, CDCl 3 )δ7.76(d, J=8.0Hz, 1H), 7.53(t, J=7.8Hz, 1H), 7.38-7.22(m, 2H), 7.18(s, 4H), 7.08(s, 2H), 6.58(s, 1H), 5.85(s, 1H), 5.30(s, 1H), 2.35(s, 3H), 1.38(s, 18H)ppm; 13 C NMR (75MHz, CDCl 3 )δ163.4, 160.6, 153.4, 152.7, 137.5, 137.1, 137.0, 131.9, 130.4, 129.8, 128.6, 125.4, 123.9, 123.1, 116.4, 116.1, 108.3, 46.9, 34.5, 30.2, 21.1ppm; m / z 471.2[M+H] + ; m.p.202-204°C.
Embodiment 2
[0021] 3-((3,5-di-tert-butyl-4-hydroxyphenyl)(4-methoxyphenyl)methyl)-4-hydroxycoumarin (2)
[0022]
[0023] Take a reaction tube, add 2,6-di-tert-butyl-4-(4-methoxybenzylidene)cyclohexa-2,5-dienone (64mg, 0.2mmol, 1.0equiv), 4- Hydroxycoumarin (38mg, 0.24mmol, 1.2equiv), boron trifluoride diethyl ether (0.04mmol, 0.2equiv), 1mL dichloromethane, reacted at room temperature for 24 hours, evaporated the solvent under reduced pressure and separated by silica gel column 85 mg of white solid product was obtained with a yield of 87%.
[0024] 1 H NMR (300MHz, CDCl 3 )δ7.74(d, J=7.9Hz, 1H), 7.52(t, J=7.7Hz, 1H), 7.40-7.14(m, 4H), 7.06(s, 2H), 6.90(d, J=8.2 Hz, 2H), 6.58(s, 1H), 5.81(s, 1H), 5.30(s, 1H), 3.80(s, 3H), 1.37(s, 18H)ppm; 13 C NMR (75MHz, CDCl 3 )δ163.4, 160.6, 158.8, 153.4, 152.7, 137.1, 132.4, 131.9, 130.6, 129.8, 125.4, 123.9, 123.1, 116.4, 116.1, 114.5, 108.3, 55.3, 46.5, 34.5, 30.2ppm; m / z 487.2[M+H] + ; m.p.217-218℃.
Embodiment 3
[0026] 3-((3,5-di-tert-butyl-4-hydroxyphenyl)(4-fluorophenyl)methyl)-4-hydroxycoumarin (3)
[0027]
[0028] Take a reaction tube, add 2,6-di-tert-butyl-4-(4-fluorobenzylidene)cyclohexa-2,5-dienone (62mg, 0.2mmol, 1.0equiv), 4-hydroxy Soybean (38mg, 0.24mmol, 1.2equiv), boron trifluoride ethyl ether (0.04mmol, 0.2equiv), 1mL dichloromethane, reacted at room temperature for 24 hours, evaporated the solvent under reduced pressure and separated through silica gel column to obtain The pink solid product is 82mg, and the yield is 86%.
[0029] 1 H NMR (300MHz, CDCl 3 )δ7.72(d, J=7.9Hz, 1H), 7.53(t, J=7.7Hz, 1H), 7.40-7.14(m, 4H), 7.04(t, J=8.2Hz, 4H), 6.52( s, 1H), 5.80 (s, 1H), 5.31 (s, 1H), 1.34 (s, 18H) ppm; 13 CNMR (75MHz, CDCl 3 )δ163.6, 160.8, 153.7, 152.7, 137.5, 136.0, 132.1, 130.4, 130.3, 125.3, 123.9, 123.2, 116.5, 116.0, 115.9, 115.6, 107.7, 46.7, 34.5, 30.1ppm; MS (ESI) z 475.2[M+H] + ; m.p.225-227℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com