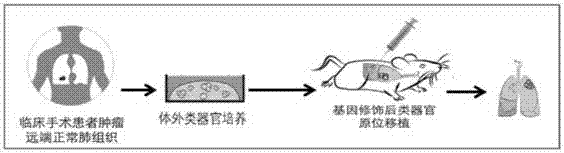
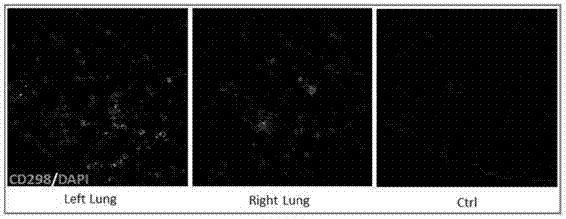
Lung and lung cancer tissue culture method and method using lung and lung cancer tissue culture method to build lung cancer mouse animal model
A lung tissue and lung cancer technology, applied in the field of biomedicine, can solve the problems such as the inability of the PDX model to provide the in situ microenvironment of the lung tissue, the loss of biological characteristics of human-derived tumors, and the inability to meet the needs of model construction, achieving a high degree of consistency and quantity. It is easy to expand and is beneficial to the effect of scientific experimental research needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Lung Tissue and Lung Cancer Tissue Organoid Culture
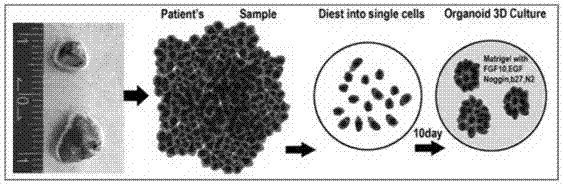
[0092] as per figure 1 The process sequence shown is for 3D culture of lung tissue / lung cancer tissue organoids. Take lung tissue and lung cancer tissue and cut them into pieces on ice, add 10ml collagenase to resuspend, and run Human Lung and Human Tumor program 1 in gentleMACS C tube collagenase. Transfer to 37°C, 220rpm shaker for digestion for 20min. The Human Lung and Human Tumor programs 2 were run using gentalMACS. Transfer to 37°C, 220rpm shaker for digestion for 10min, filter the cells with a 100μm cell mesh, add 10ml DMEM / F12 to the filtrate to stop digestion, centrifuge (4°C, 200g, 5min), and remove the supernatant.
[0093] Take 5ml of erythrocyte lysate to resuspend, and lyse the erythrocytes on ice for 5min. In a centrifuge at 4°C, centrifuge at 200g for 5min, and remove the supernatant. Add 10ml DMEM / F12 to resuspend, centrifuge at 200°C for 5min at 4°C, and remove the supernatant. Obtain indep...
Embodiment 2
[0104] Lung Tissue and Lung Cancer Tissue Organoid Culture
[0105] Take lung tissue and lung cancer tissue and cut them into pieces on ice, add 10ml collagenase to resuspend, and run Human Lung and Human Tumor program 1 in gentleMACS C tube collagenase. Transfer to 37°C, 220rpm shaker for digestion for 15min. The Human Lung and Human Tumor programs 2 were run using gentalMACS. Transfer to 37°C, 220rpm shaker for digestion for 10min, filter the cells with a 100μm cell mesh, add 10ml DMEM / F12 to the filtrate to stop digestion, centrifuge (4°C, 200g, 6min), and remove the supernatant.
[0106] Take 5ml of erythrocyte lysate to resuspend, and lyse the erythrocytes on ice for 6min. In a centrifuge at 4°C, centrifuge at 200g for 5min, and remove the supernatant. Add 10ml DMEM / F12 to resuspend, centrifuge at 200°C for 7min at 4°C, and remove the supernatant.
[0107] For cell counting, mix Martrigel, 20,000 cells per 40 μL, drop in the center of a 64-well plate, place the cult...
Embodiment 3
[0111] Organoid Passaging
[0112] Collect the organoids in the culture dish of Example 2, resuspend the organoids in 3ml TrypLE, and digest at 37°C for 10min. Add 5ml DMEM / F12 to stop the digestion, centrifuge at 200g, 4°C for 5min, add an appropriate amount of Martrigel to resuspend according to the amount of cells, and drop in the center of the well of a 48-well plate. Place the Petri dish at 37°C (5% CO 2 ) 10min, solidify the Martrigel. Then, 150 μL of conditioned medium was added to each well at 37°C, 5% CO 2 Cell culture incubator. Change the medium every 2-3 days. Obtain passaged human lung tissue and lung cancer tissue passaged organoids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com