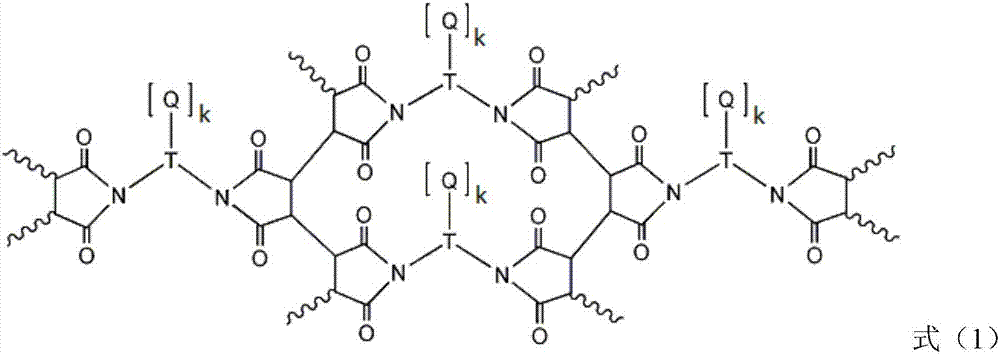
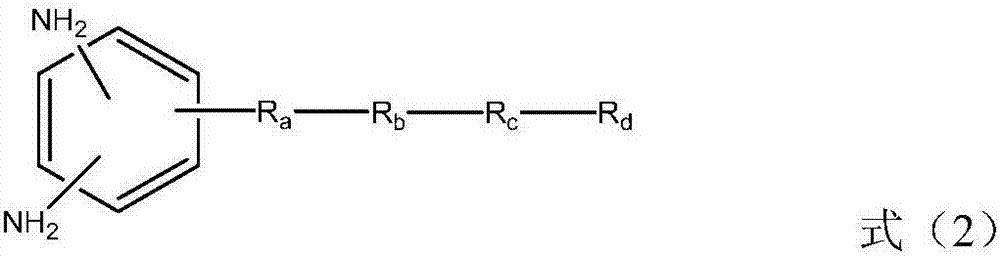
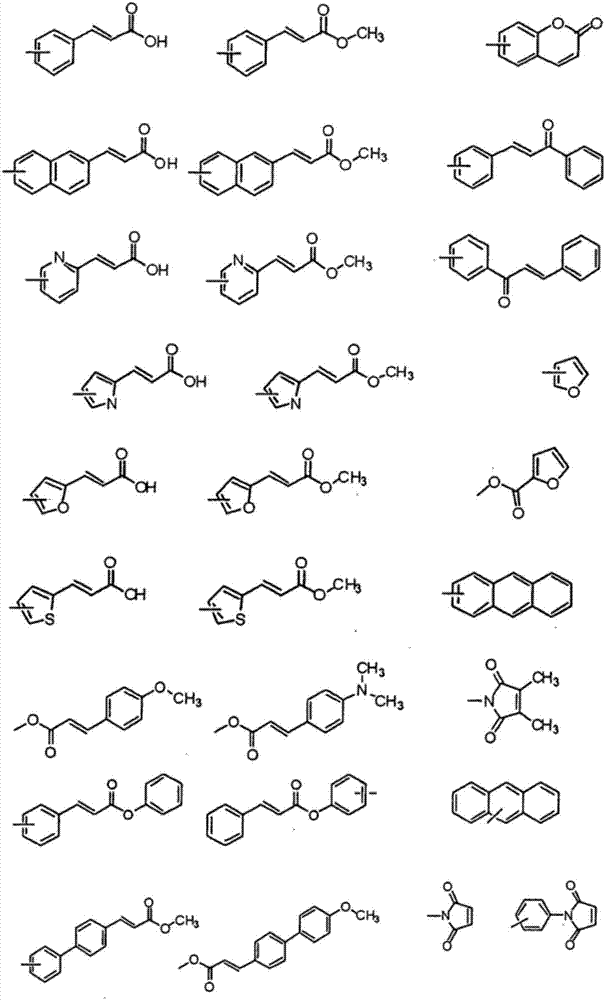
Liquid crystal alignment film, liquid crystal display element and manufacturing method of the same
A technology of liquid crystal alignment and maleamic acid group, which is applied in the direction of liquid crystal materials, chemical instruments and methods, instruments, etc., can solve the problems of unreacted compounds remaining in the liquid crystal layer, failure to reach a practical level, liquid crystal molecule decomposition, etc., to achieve Long-term reliable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] In formula (2), R a Indicates -CH 2 -, -O-, -NH-, -N(CH 3 )-, -CONH-, -NHCO-, -CH 2 O-, -COO-, -OCO-, -CON(CH 3 )-or-N(CH 3 ) CO-. R a Can be formed by general organic synthesis methods, but from the viewpoint of ease of synthesis, -CH 2 -, -O-, -NH-, -NHCO-, -CH 2 O- or -COO- is preferred. R b Represents an unsubstituted or fluorine atom-substituted linear alkylene group with a carbon number of 1 to 20, and any -CH in the alkylene group 2 -Available by-CF 2 -, -CH=CH- or the following substituents are substituted, and the substituents include: -O-, -COO-, -NHCO-, -NH-, carbocyclyl or heterocyclyl, wherein, when two -CH above 2 - When substituted by the substituent, the substituent is not adjacent. Preferably, R b Represents a linear alkylene group with a carbon number of 1 to 20, more preferably, R b Represents a linear alkylene group having 2 to 8 carbon atoms.
[0058] The carbocyclyl may include the following structures, but is not limited thereto. ...
Synthetic example
[0188] Synthesis Example A-1 to Synthesis Example A-12 of the compound (A) having a maleamic acid group are described below:
Synthetic example A-1
[0190] A nitrogen inlet, a stirrer, a heater, a condenser tube, and a thermometer were arranged on a four-necked flask with a capacity of 500 milliliters, and nitrogen was introduced. Then, in a four-necked flask, add 3.95 grams (0.015 moles) of diamine compound Da1 (abbreviated as a2-1-1), 2.82 grams (0.0075 moles) of 1-octadecyloxy-2,4-di Aminobenzene (abbreviated as a2-2-1) and 50 grams of tetrahydrofuran (hereinafter referred to as THF), and stirred at room temperature until dissolved. Then, 2.81 grams (0.025 moles) of 2-methylmaleic anhydride (abbreviated as a1-1) was added, and reacted at room temperature for 3 hours. After the reaction, the reaction solution was filtered, and the filtered solid was repeatedly washed with THF After washing and filtering three times, placing in a vacuum oven and drying at a temperature of 60° C., the compound (A-1) having a maleamic acid group can be obtained.
[0191] Synthesis Example A-2 to Synthesis Example A-12 and Comparative Synth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com