Method for normal-pressure phosphoric-acid leaching of nickel-cobalt and synchronous preparation of iron phosphate
A technology of laterite nickel ore and iron phosphate, applied in chemical instruments and methods, phosphorus compounds, process efficiency improvement and other directions, can solve the problems of large material loss, low nickel leaching, poor nickel-iron leaching selectivity, etc., and achieve small material loss , High leaching rate, the effect of increasing the added value of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The laterite nickel ore was leached in 3mol / L phosphoric acid solution for 10h, the liquid-solid ratio was 10:1, and the leaching temperature was 80°C.
[0049] The leaching rate of nickel is 95.97%, that of cobalt is 87.61%, and that of iron is 12.19%. The nickel-iron leaching rate ratio is 7.87. The main chemical components of the leaching residue are shown in Table 2
[0050] Table 2 Main chemical composition of leaching residue / %
[0051] TF SiO 2
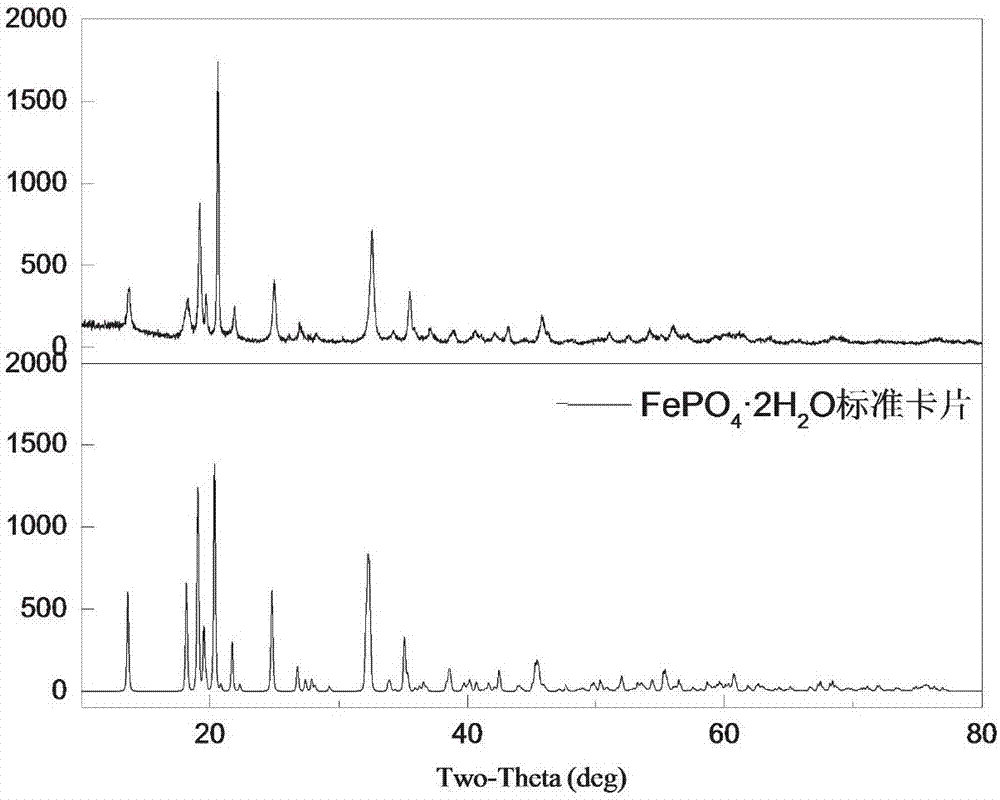
[0052] The phase analysis diagram of the leaching slag of this embodiment is shown in figure 1 ,Depend on figure 1 It can be seen that the leaching slag phase is FePO 4 2H 2 O.
Embodiment 2
[0054] The lateritic nickel ore was roasted at 400°C for 1 hour, and the calcined sand was leached in 3mol / L phosphoric acid solution for 2 hours, the liquid-solid ratio was 10:1, and the leaching temperature was 80°C.
[0055] The leaching rate of nickel was 98.13%, that of cobalt was 87.69%, and that of iron was 9.39%. The nickel-iron leaching rate ratio is 10.45
Embodiment 3
[0057] The laterite nickel ore was roasted at 400°C for 1 hour, and the calcined sand was leached in 3mol / L phosphoric acid solution for 3 hours, the liquid-solid ratio was 10:1, and the leaching temperature was 80°C.
[0058] The leaching rate of nickel is 98.43%, that of cobalt is 89.69%, and that of iron is 7.08%. The nickel-iron leaching rate ratio is 13.90. The main chemical components of the leaching residue are shown in Table 3
[0059] Table 3 Main chemical composition of leaching slag / %
[0060]
[0061]
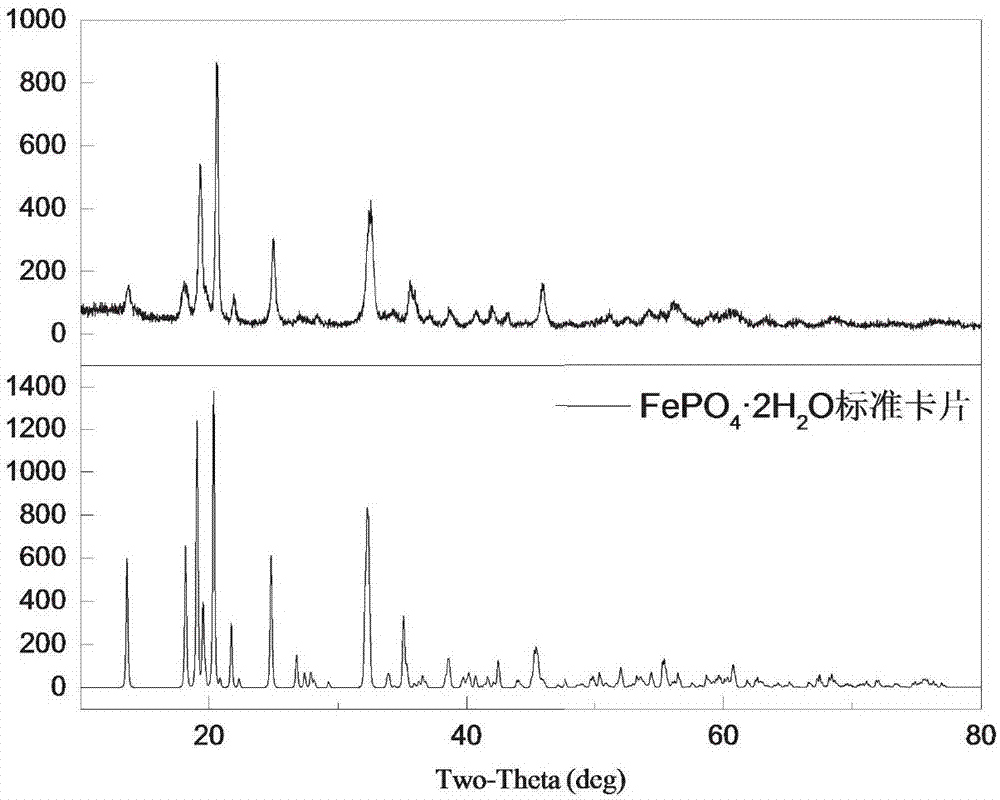
[0062] The phase analysis diagram of the leaching slag of this embodiment is shown in figure 2 ,Depend on figure 2 It can be seen that the leaching slag phase is FePO 4 2H 2 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com