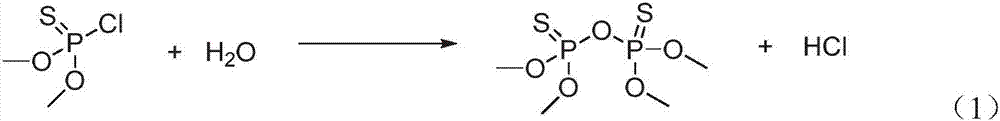
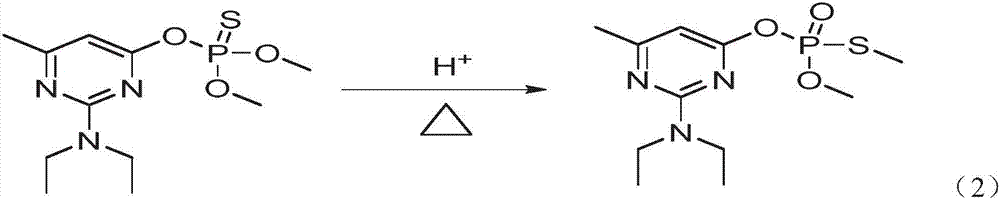
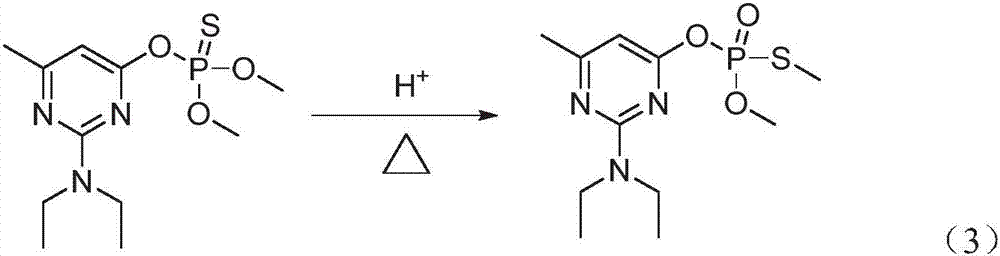
Method of controlling harmful impurities during pirimiphos-methyl synthesis
A technology of pirimiphos-methyl and harmful impurities, which is applied in the field of control of harmful impurities in the synthesis of pirimiphos-methyl, can solve problems such as failure to meet the FAO standards, achieve the speed of the main reaction, avoid product isomerization, and reduce the reaction temperature Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for controlling harmful impurities in the synthesis of pirimiphos-methyl of the present invention, the process is as follows:
[0040] Add 93.3g (0.5mol) 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine (97%), 300mL toluene and 20.4g (0.50mol) sodium hydroxide (98%), stirred at room temperature for 2h, filtered, and the filter cake was dried to obtain 101g white 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine sodium salt .
[0041] Add 101g white 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine sodium salt, 0.2g DMAP catalyst and 200mL toluene to a 1000mL three-necked flask with cooling tube, dropping funnel, thermometer, and stirring, 80.8 g of 99% O, O-dimethylphosphorylthiochloride was added dropwise under stirring at 20°C, and the addition was completed within 4 hours and the reaction was continued for 1 hour. After the reaction was completed, 200 mL of water was added and stirred, and the phases were separated. Add 200mL of water to the oil layer and st...
Embodiment 2
[0043] A method for controlling harmful impurities in the synthesis of pirimiphos-methyl of the present invention, the process is as follows:
[0044] Add 93.3g (0.5mol) 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine (97%), 300mL toluene and 22.4g (0.55mol) sodium hydroxide (98%), stirred at room temperature for 2h, filtered, and the filter cake was dried to obtain 103.1g white 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine sodium Salt.
[0045] Add 103.1g white 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine sodium salt, 0.2g DMAP catalyst and 300mL toluene to a 1000mL three-neck flask with cooling tube, dropping funnel, thermometer, and stirring , 78.4g of 99% O, O-dimethylphosphorylthiochloride was added dropwise under stirring at 20°C, and the addition was completed within 3 hours and the reaction was continued for 1h. After the reaction was completed, 200mL of water was added and stirred, and the phases were separated. Add 200mL water to the oil layer and stir, let...
Embodiment 3
[0047] A method for controlling harmful impurities in the synthesis of pirimiphos-methyl of the present invention, the process is as follows:
[0048] Add 93.3g (0.5mol) 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine (97%), 300mL toluene and 32.1g (0.55mol) potassium hydroxide (96%), stirred at room temperature for 2h, filtered, and the filter cake was dried to obtain 113.2g white 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine potassium Salt.
[0049] Add 112.2g white 2-N, N-diethylamino-6-methyl-4-hydroxypyrimidine sodium salt, 0.1g DMAP catalyst and 300mL toluene to a 1000mL three-necked flask with cooling tube, dropping funnel, thermometer and stirring 80.8 g of 99% O, O-dimethylphosphoryl thiochloride was added dropwise under stirring at 15°C, and the addition was completed within 2 hours and the reaction was continued for 1 hour. After the reaction was completed, 200 mL of water was added and stirred, and the phases were separated. Add 200mL of water to the oil laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com