Application of non-peptide small-molecular compound as MC4R (melanocortin-4 receptor) agonist and specific mutant medicine mate thereof
A compound and mutant technology, applied in the field of drug preparation and application, can solve problems such as cell membrane localization defects, pERK1/2 signal defects, and ligand affinity defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Basic preparation example
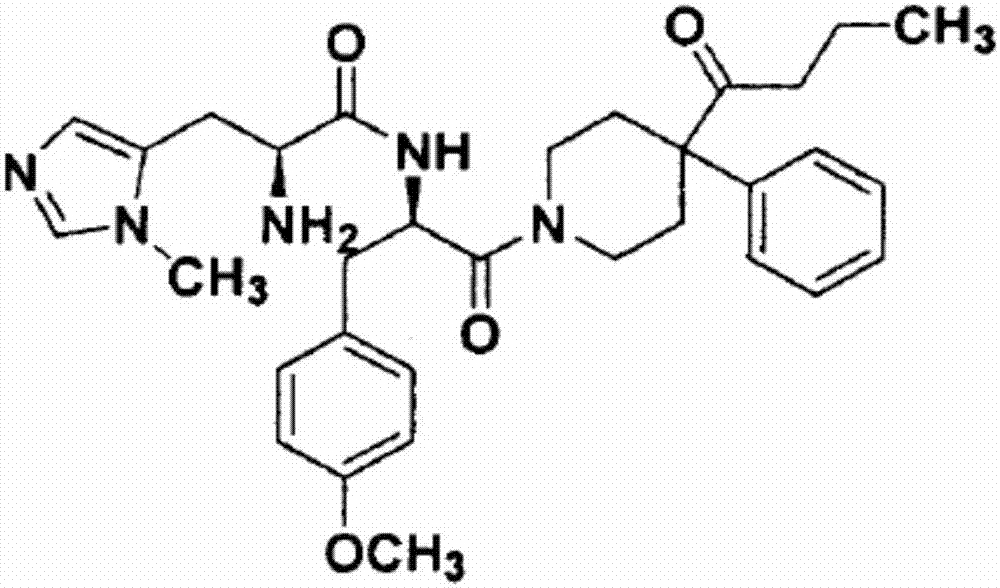
[0026] The present invention selects commercial plasmid pcDNA3.1(+) (purchased from Clontech Company) as an expression vector, and constructs an expression vector of wild-type (WT) human MC4R (hMC4R): pcDNA3.1-hMC4R-WT. That is, an expression sequence (NM_005912.2) encoding the wild-type hMC4R gene was inserted into the multiple cloning site of pcDNA3.1(+).
[0027] The present invention uses the constructed pcDNA3.1-hMC4R-WT as a template, and utilizes a rapid site-directed mutagenesis kit (purchased from Stratagene) to construct 20 genes named N62S, I69R, P78L, C84R, G98R, Y157S, M161T, W174C, P260Q, C271Y, G55V, Δ88-92, I102T, L106P, D126Y, A219V, D90N, S136F, A175T, C326R Functionally identified expression vectors for the second, third and fourth types of MC4R mutants.
[0028]In the present invention, human kidney embryonic cells (HEK293T) not expressing MC4R receptors are used as a transient expression system for MC4R and mut...
Embodiment 2
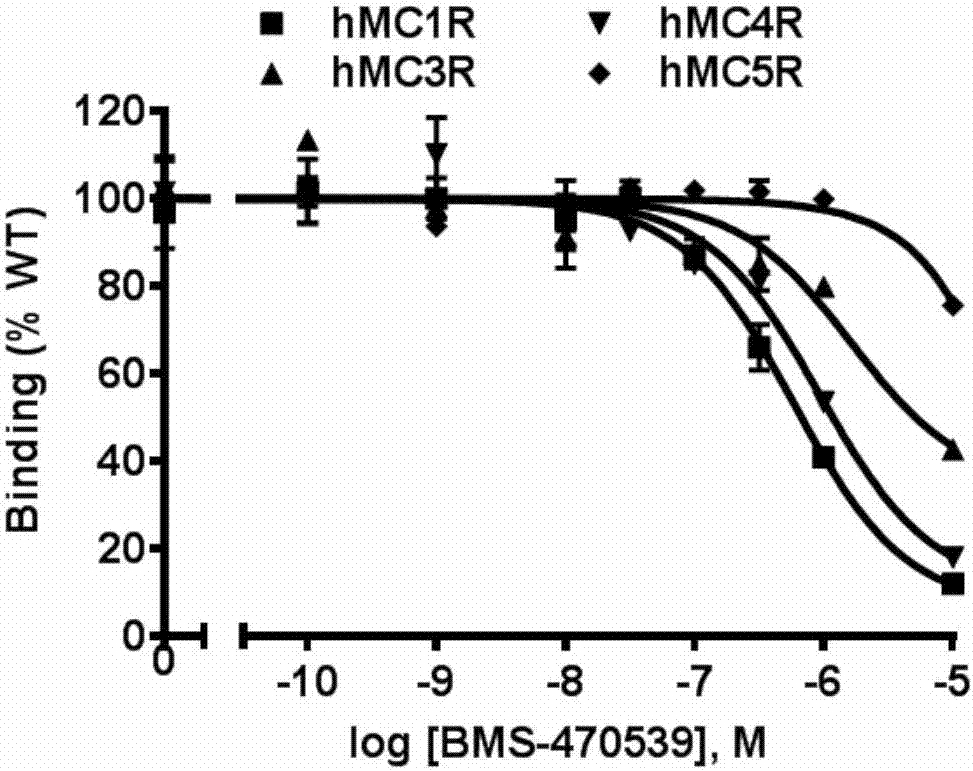
[0031] Example 2 Detection of affinity between BMS-470539 and MC1R, MC3R, MC4R, MC5R
[0032] Before HEK293T cells were collected, the cells were washed twice with serum-free medium (purchased from Gibco), and different final concentrations (10 -10 ~10 -5 M) Ligand BMS-470539 (purchased from Bachem Company) and 100,000 cpm radiolabeled ligand 125 HEK293T cells (purchased from ATCC) transfected with MC1R, MC3R, MC4R and MC5R were co-incubated with I-NDP-MSH (purchased from the Peptide Radioactive Iodine Labeling Service Center of the University of Mississippi) (50 μL) at 37°C for 1 h, and after the reaction was terminated, Pre-cooled phosphate buffered saline (PBS (137mM NaCl, 2.7mM KCl, 1.4mM KH 2 PO 4 , 4.3 mM Na 2 HPO 4 , pH 7.4) to wash away unbound markers, add 100 μL of 0.5N NaOH to collect the cells, and use a gamma scintillation counter to measure the amount of receptor-bound cells in the cells 125 The total amount of I-NDP-MSH, that is, the binding ability of MC1...
Embodiment 3
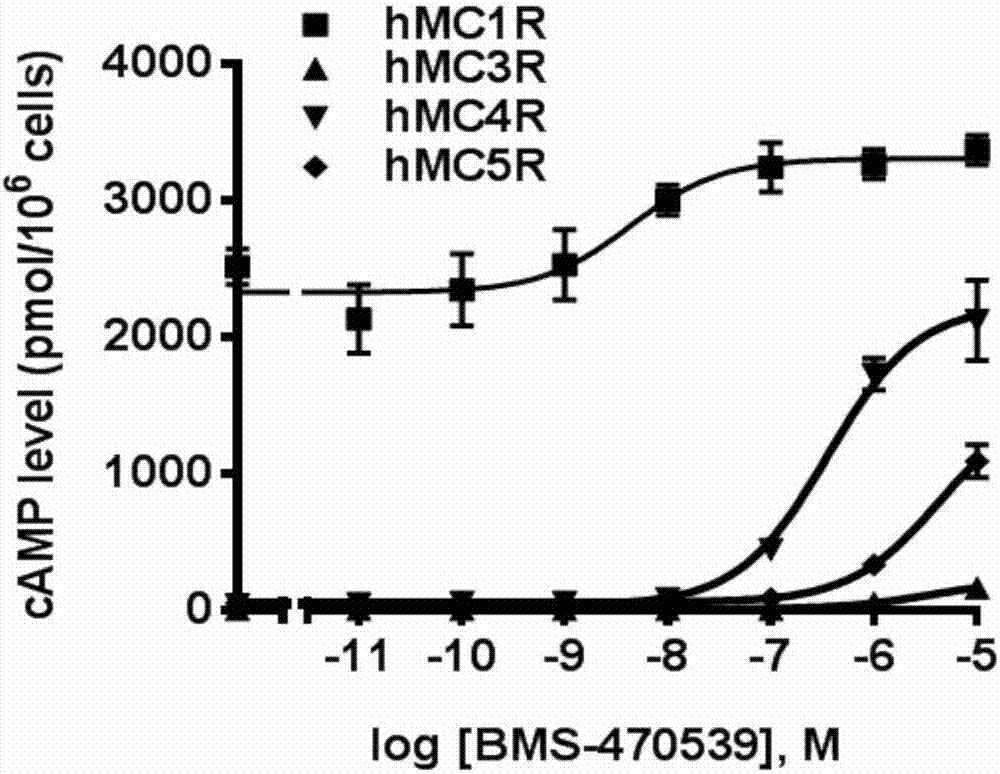
[0037] Example 3 Detection of the ability of BMS-470539 to induce MC1R, MC3R, MC4R and MC5R to produce signal molecule cAMP
[0038] Before HEK293T cells were collected, the cells were washed twice with serum-free medium, and the serum-free medium (purchased from Gibco) containing 0.5 mM isobutylmethylxanthine was incubated at 37°C for transfected MC1R, MC3R, HEK293T cells of MC4R and MC5R for 15min, and then different final concentrations (10 -10 ~10 -5 M) The cells were stimulated with BMS-470539, incubated at 37°C for 1 hour, put the cell culture plate on ice, discarded the culture medium, added 0.5N perchloric acid containing 180 μg / mL theophylline to extract intracellular cAMP, and used radiation Immunoassay (RIA) method 22 The dose-dependent determination of the signal molecule cAMP was carried out, and its effective intermediate concentration (EC 50 ) and the maximum concentration value (R max ).
[0039] The measurement results of this example are consistent with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com