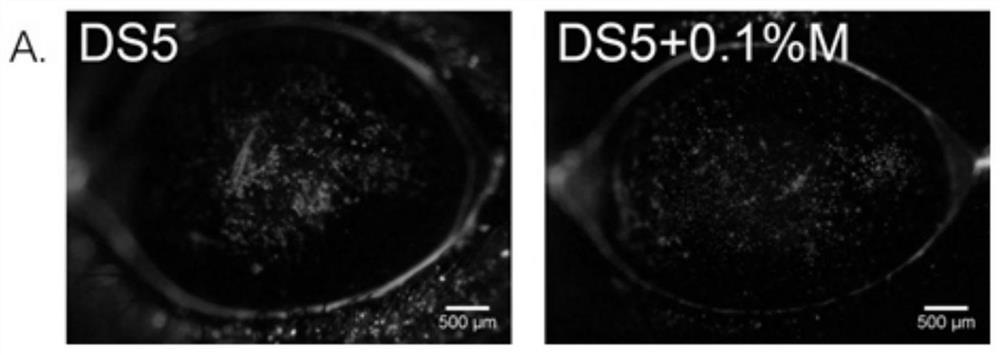
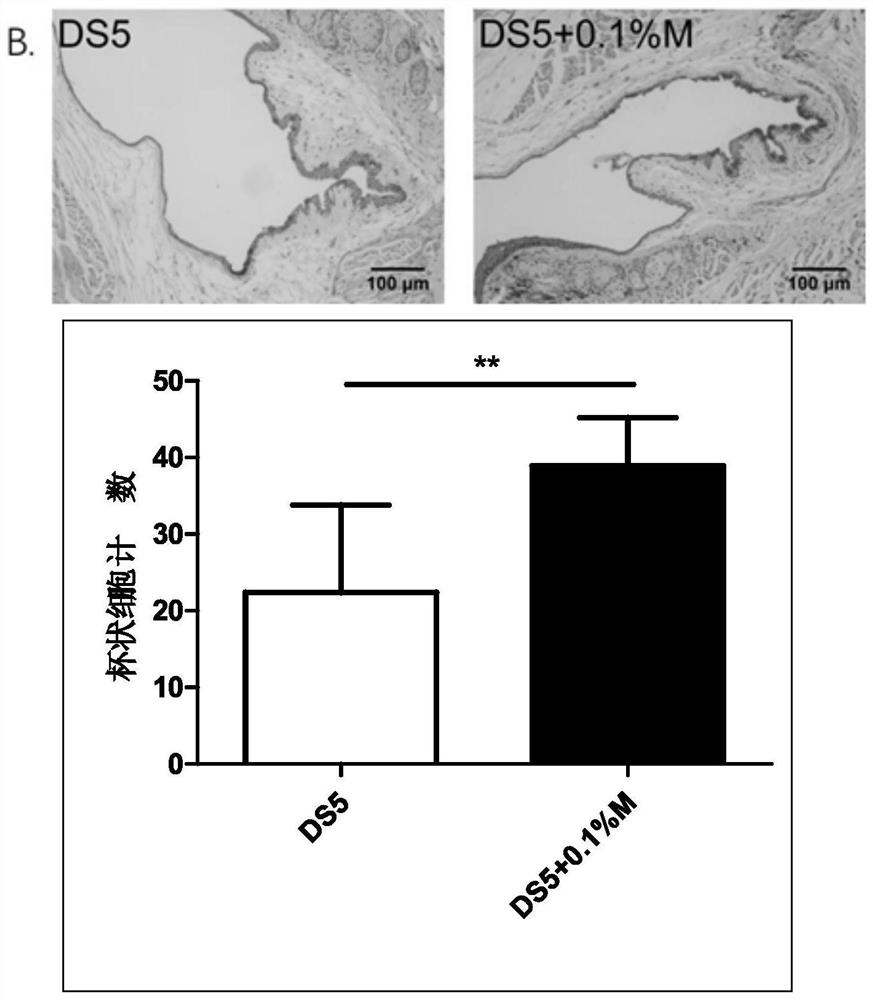
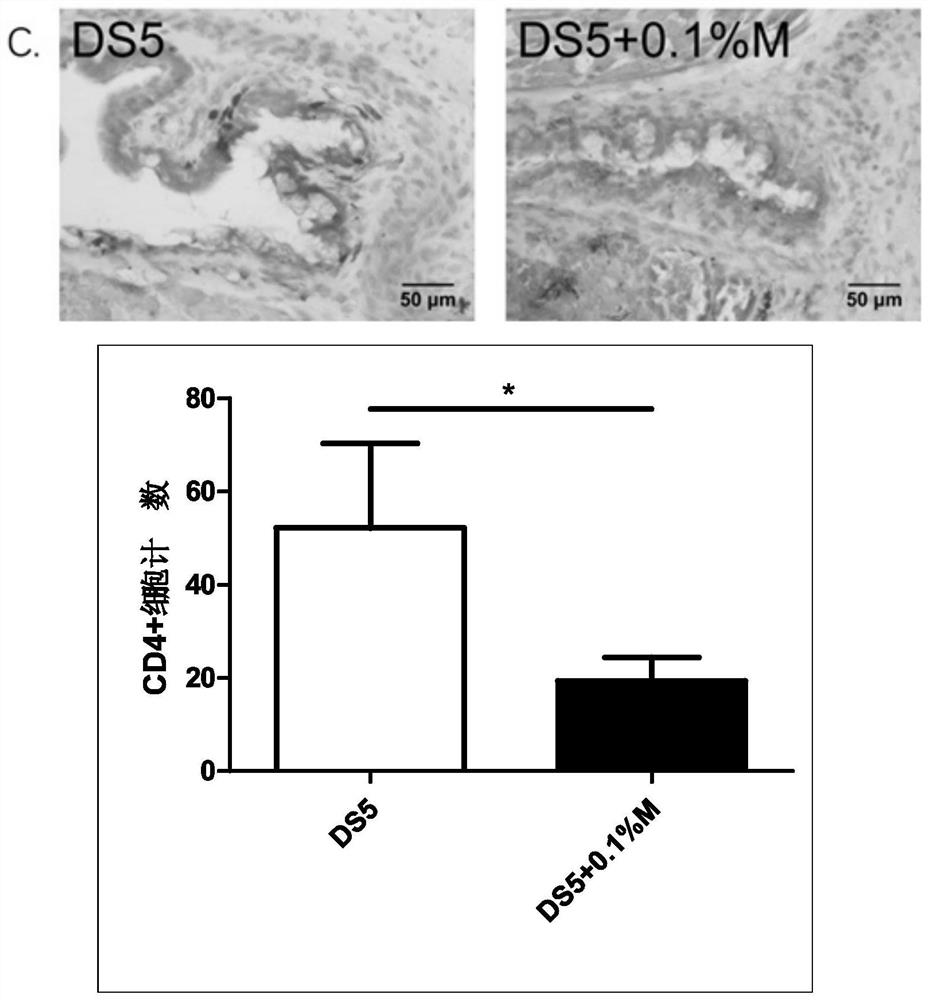
An eye drop for the treatment of dry eyes
一种滴眼液、干眼的技术,应用在非有效成分的医用配制品、含有效成分的医用配制品、无机非有效成分等方向,能够解决脂溶性滴眼液先天不足无法完全克服等问题,达到保护角膜上皮屏障功能、好抗炎及免疫抑制效果、恢复数量的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] formula:
[0023]
[0024] Preparation Process:
[0025] 1. Accurately weigh 1.00g of midazoribine, 0.03g of benzalkonium chloride, 8.30g of sodium chloride, 30mL of 0.1mol / L hydrochloric acid, add 200mL of water for injection to dissolve, stir evenly, and dilute to 1000mL with water for injection to obtain liquid medicine.
[0026] 2. Filter the above solution once through a 0.45 μm microporous membrane, and then twice through a 0.22 μm microporous membrane.
[0027] 3. After the medicinal solution is qualified, fill 5.0mL of the above medicinal solution into a 5mL single-dose packaging plastic container in a grade A environment, and seal it to obtain the finished product.
Embodiment 2
[0029] formula:
[0030]
[0031] Preparation Process:
[0032] 1. Accurately weigh 1.00g of midazoribine, add 300mL of water for injection to dissolve it, and obtain solution ① for later use.
[0033]2. Accurately weigh 0.05g of chlorhexidine acetate, 7.20g of sodium dihydrogen phosphate, 0.95g of disodium hydrogen phosphate, and 4.79g of sodium chloride, add 200mL of water for injection to dissolve, stir and mix evenly to obtain a solution② spare.
[0034] 3. Mix solution ① and solution ②, stir evenly, and dilute to 1000mL with water for injection to obtain a medicinal solution.
[0035] 4. Filter the above medicinal solution once through a 0.45 μm microporous membrane, and then twice through a 0.22 μm microporous membrane.
[0036] 5. After the medicinal solution is qualified, fill 5.0mL of the above medicinal solution into a 5mL single-dose packaging plastic container in a grade A environment, and seal it to obtain the finished product.
Embodiment 3
[0038] formula:
[0039]
[0040] Preparation Process:
[0041] 1. Accurately weigh 1.00g midazoribine, 0.03g benzalkonium bromide, 6.40g sodium dihydrogen phosphate, 1.89g disodium hydrogen phosphate, 4.72g sodium chloride, 0.10g disodium edetate, add 200mL Dissolve in water for injection, stir evenly, and dilute to 1000mL with water for injection to obtain a medicinal solution.
[0042] 2. Filter the medicinal solution prepared above through a 0.45 μm microporous membrane once, and then through a 0.22 μm microporous membrane once.
[0043] 3. The filtered liquid medicine is sterilized at 121° C. for 8 minutes by autoclaving, and then cooled.
[0044] 4. After the medicinal solution is qualified, fill 5.0mL of the above medicinal solution into a 5mL single-dose packaging plastic container in a Class A environment, and seal it to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com