Dna vaccine composition for preventing and treating herpes zoster, and method for activating t cells for vzv antigen by using same
A DNA vaccine, a technology for herpes zoster, which is applied in the field of vaccines for the prevention and treatment of herpes zoster, can solve the problems of patients who cannot be immunosuppressed by herpes zoster, and achieves remarkable in vivo delivery efficiency, easy mass production, and improved stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
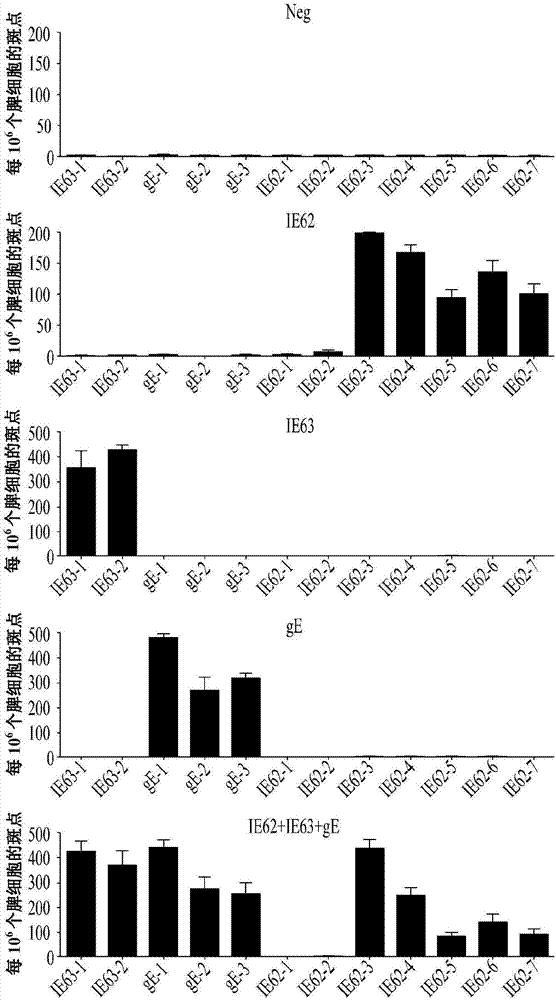
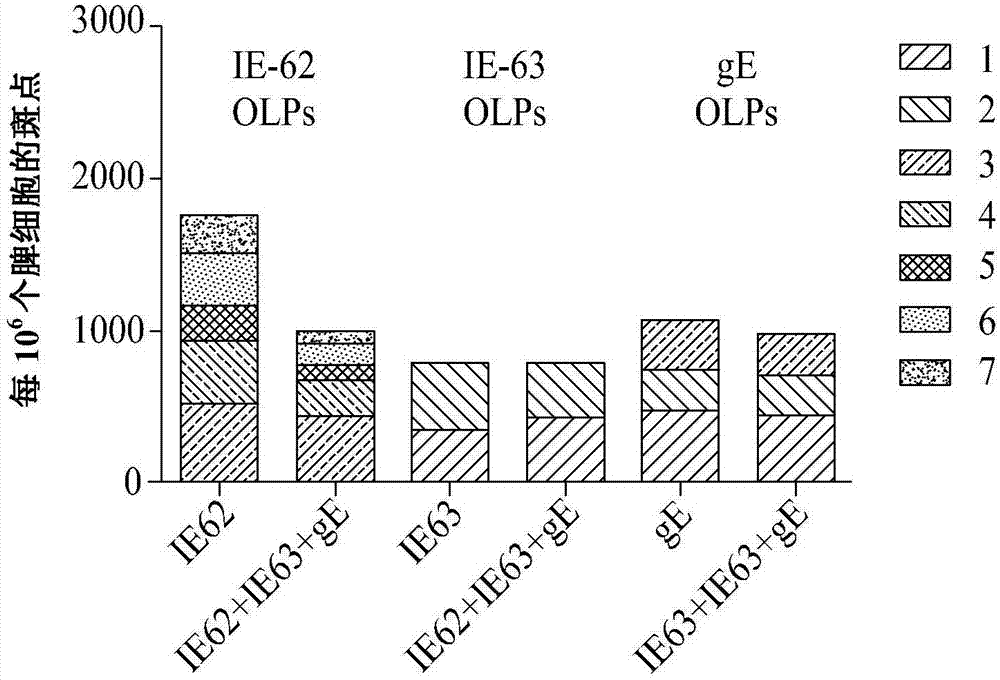
[0026] According to an exemplary embodiment of the present invention, the protein may include protein IE-62 (SEQ ID NO: 1) and protein IE-63 (SEQ ID NO: 2) and glycoprotein gE (SEQ ID NO: 3), Both of them can induce cellular immune response, and the plasmid is designed so that the gene encoding this protein can be inserted into the plasmid.
[0027] Administration of the plasmid DNA allows the plasmid DNA to mix with fluids in the body. For this reason, the plasmid DNA of the present invention can be designed to be introduced into the body with high efficiency using electroporation. Only when this method of electroporation is used, in vivo delivery efficiency can be maximized, thereby maximizing therapeutic efficacy. Body parts to which plasmid DNA is administered by electroporation include muscle parts and the like, but the present invention is not particularly limited thereto. For example, the body part can be determined in consideration of various factors such as age, typ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com