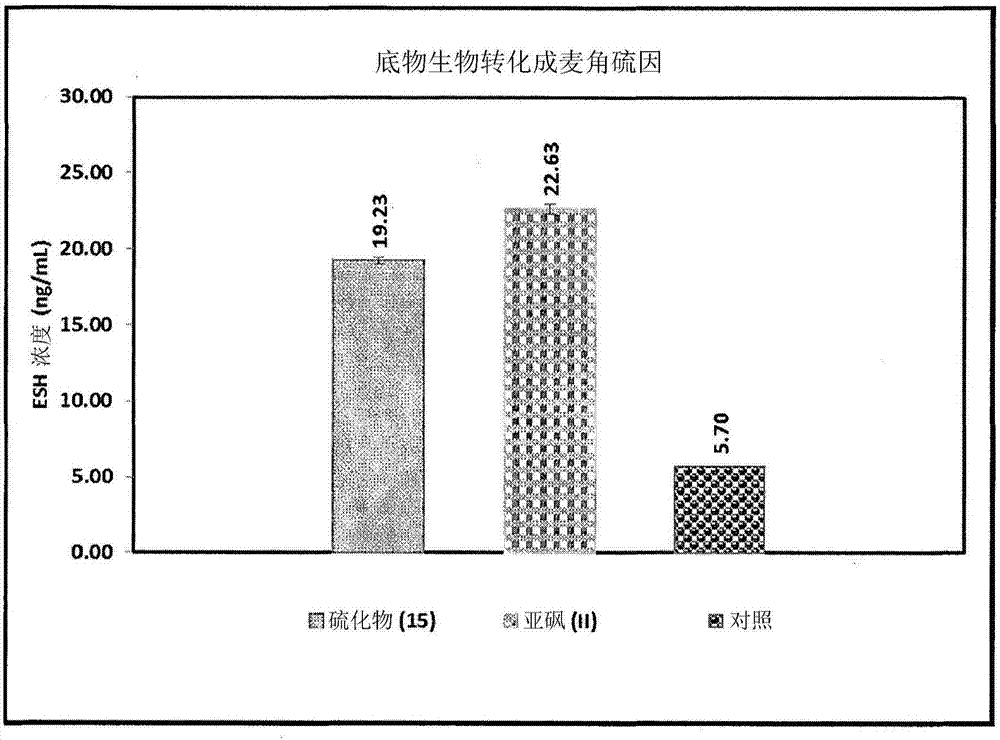
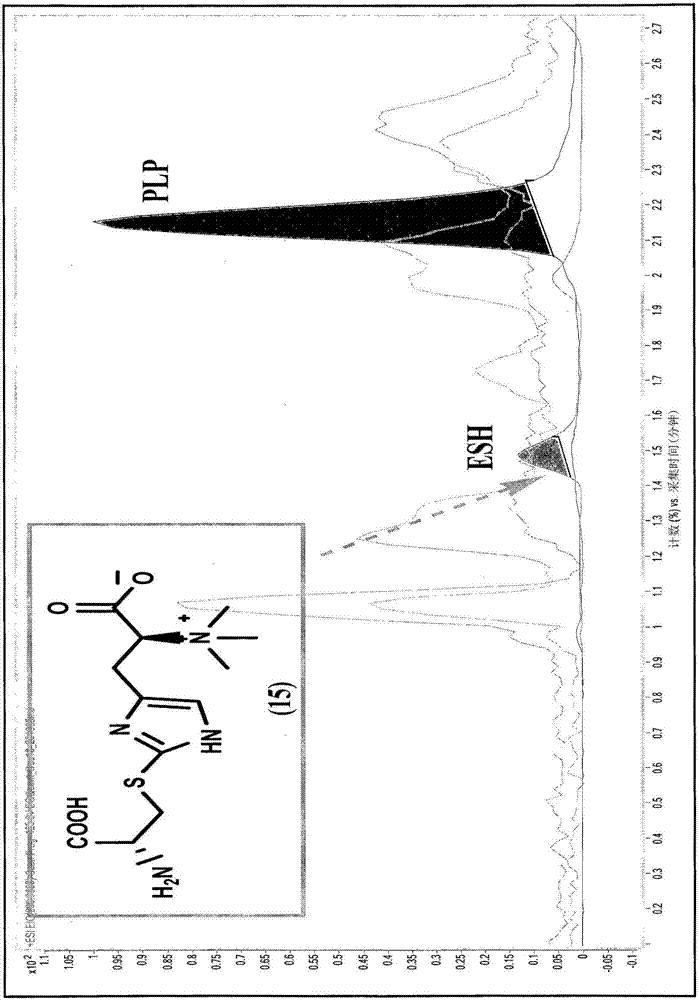
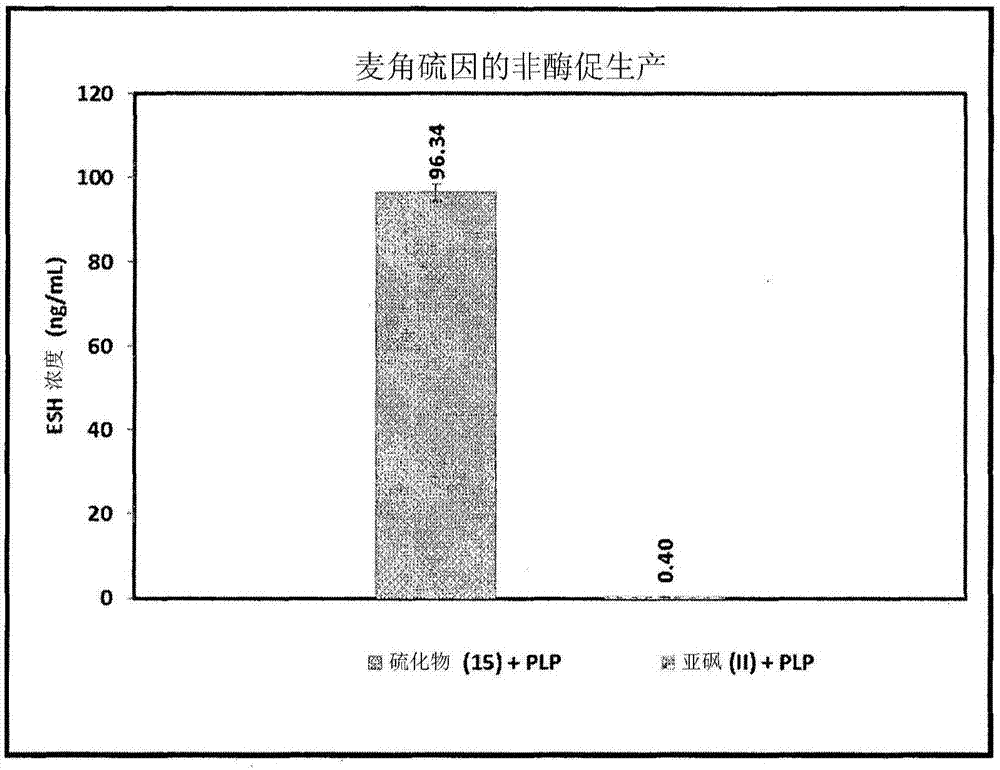
Process for synthesizing ergothioneine and related compounds
A kind of technology of ergothioneine and compound, applied in the field of synthesizing ergothioneine and related compounds, can solve problems such as high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0139] 1. General procedure
[0140] All solvents were dried by appropriate techniques and freshly distilled before use. All commercially available reagents were purchased from Sigma-Aldrich and Merck and used without further purification.
[0141] Unless otherwise stated, the reactions were carried out in oven-dried glassware under an inert atmosphere of nitrogen, and the 254 Thin layer chromatography (TLC) monitoring performed on precoated plates (0.2 mm layer), product visualized under UV light at 254 nm or by spraying the plate with ninhydrin (2% v / v) in ethanol and then visualized by heating .
[0142] Column chromatography was performed by using Merck Kieselgel silica gel 60 (0.040mm-0.063mm) and eluted with a suitable solvent mixture. All compounds were dried under vacuum before determining their yields.
[0143] Unless otherwise stated, NMR spectra ( 1 H and 13 C) Recorded at Varian Mercury 300MHz (75MHz, 13 C), Varian Unity 400MHz (101MHz, 13 C), Bruker unity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com