A kind of fluorescent probe containing selenium atom and its preparation method and application
A technology of fluorescent probes and ions, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of sensitivity and high selectivity, ease of use and low detection limit, poor practicability of detection means, sensor Complex structure and other issues, to achieve the effect of low detection limit, mild conditions and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
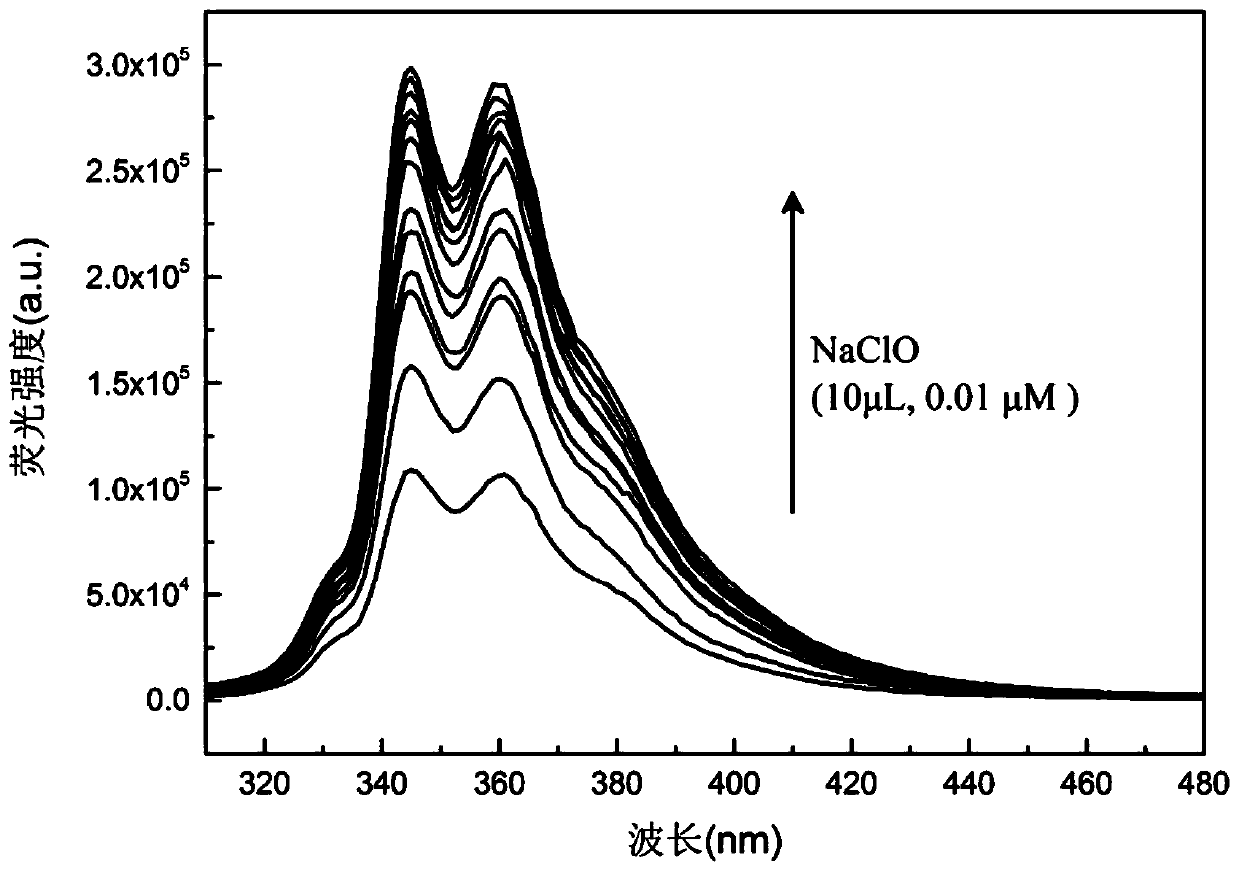
Image
Examples
Embodiment 1
[0031] A fluorescent probe containing a selenium atom, the structure is as shown in formula (I):
[0032]
[0033] Wherein, Ph is a phenyl group, and Ar is a carbazole or diphenylamine group, as shown in (II):
[0034]
[0035] x=1 or 2, y=3-x.
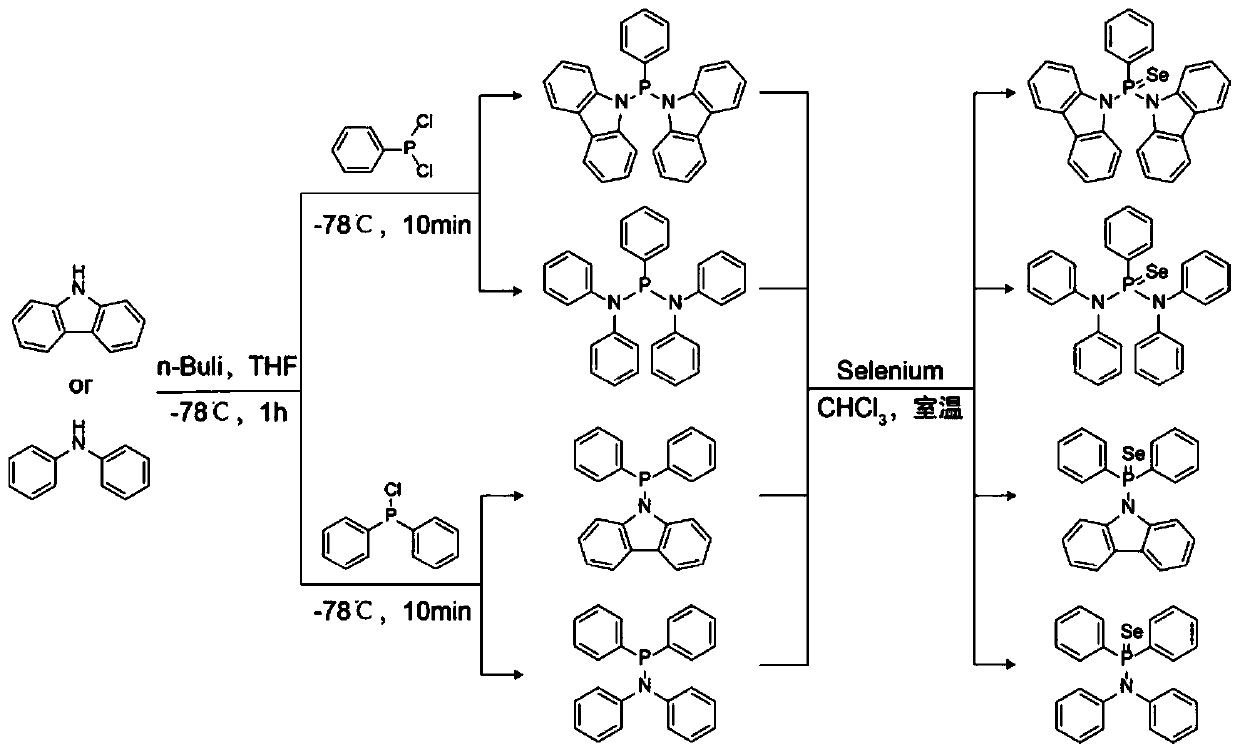
[0036] The synthetic route of the fluorescent probe containing selenium atom is shown in figure 1 .
[0037] Concrete synthetic steps are as follows:
[0038] Step 1. Under the protection of nitrogen, using tetrahydrofuran as a solvent, react n-butyllithium with carbazole or diphenylamine at -78°C for 1 hour to form an organolithium compound system, wherein carbazole or diphenylamine and n-butyllithium The molar ratio is 1:1;
[0039] Step 2. Under the protection of nitrogen, add phenylphosphine dichloride or diphenylphosphine chloride to the organolithium compound system described in step 1 and react at room temperature for 4h to obtain a derivative containing N-P structure, wherein phenylphosphine dichloride The molar rat...
Embodiment 2
[0043] A fluorescent probe containing a selenium atom, the structure is as shown in formula (I):
[0044]
[0045] Wherein, Ph is a phenyl group, and Ar is a carbazole or diphenylamine group, as shown in (II):
[0046]
[0047] x=1 or 2, y=3-x.
[0048] The synthetic route of the fluorescent probe containing selenium atom is shown in figure 1 .
[0049] Concrete synthetic steps are as follows:
[0050] Step 1. Under the protection of nitrogen, using tetrahydrofuran as a solvent, react n-butyllithium with carbazole or diphenylamine at -78°C for 2 hours to form an organolithium compound system, wherein carbazole or diphenylamine and n-butyllithium The molar ratio is 2:1;
[0051] Step 2. Under the protection of nitrogen, add phenylphosphine dichloride or diphenylphosphine chloride to the organolithium compound system described in step 1 and react at room temperature for 12 hours to obtain a derivative containing N-P structure, wherein phenylphosphine dichloride The mo...
Embodiment 3
[0055] A fluorescent probe containing a selenium atom, the structure is as shown in formula (I):
[0056]
[0057] Wherein, Ph is a phenyl group, and Ar is a carbazole or diphenylamine group, as shown in (II):
[0058]
[0059] x=1 or 2, y=3-x.
[0060] The synthetic route of the fluorescent probe containing selenium atom is shown infigure 1 .
[0061] Concrete synthetic steps are as follows:
[0062] Step 1. Under the protection of nitrogen, using tetrahydrofuran as a solvent, react n-butyllithium with carbazole or diphenylamine at -78°C for 1 hour to form an organolithium compound system, wherein carbazole or diphenylamine and n-butyllithium The molar ratio is 1.5:1;
[0063] Step 2. Under the protection of nitrogen, add phenylphosphine dichloride or diphenylphosphine chloride to the organolithium compound system described in step 1 and react at room temperature for 8h to obtain a derivative containing N-P structure, wherein phenylphosphine dichloride The molar rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com