Pteridinone derivatives and applications as flt3 inhibitors
A technology of medicine and use, applied in pteridone derivatives, the application field of medicine, can solve problems such as unsatisfactory clinical effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] 1) Synthesis of 8-(4-aminophenyl)-2-(4-methoxy-2-methylphenyl)-7(8H)-pteridinone (F-1)
[0144] ① Synthesis of (4-aminophenyl) tert-butyl carbamate
[0145]
[0146] Weigh p-phenylenediamine (890mg, 8.23mmol) and put it in a 100mL round bottom flask, add 40mL of dichloromethane, stir under ice bath, and take di-tert-butyl dicarbonate (449mg, 2.06mmol) and dissolve it in dichloromethane In the middle, it was added dropwise to the above reaction solution, and then continued to stir overnight at room temperature. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was separated by silica gel column chromatography (petroleum ether / ethyl acetate=3:1, v / v) to obtain 398 mg of tert-butyl (4-aminophenyl)carbamate, with a yield of 93 %.
[0147] 1 H NMR (400MHz, CDCl 3 ): δ7.14(d, J=8.0Hz, 2H), 6.65(d, J=8.4Hz, 2H), 6.26(s, 1H), 1.52(s, 9H). Melting point 112-113℃, literature value [97] 112-114°C.
[0148] ②Synthesis of...
Embodiment 2
[0227] The synthesis steps of compound 1 are as follows, and the synthesis of compound 2-31 is carried out with reference to the synthesis of compound 1:
[0228] Synthesis of (S)-3-((2-chloro-5-nitropyrimidine)4-amino)pyrrolidine-1-carboxylic acid tert-butyl ester
[0229]
[0230] Weigh 2,4-dichloro-5-nitropyrimidine (3.23g, 16.28mmol) into a 250mL low-temperature reaction flask, add 80mL of dichloromethane to dissolve, stir and cool at -70°C, and take another (S)-1 - tert-butoxycarbonyl-3-aminopyrrolidine (3.03g, 16.28mmol), N,N-diisopropylethylamine (2.52g, 19.53mmol) were dissolved in 20mL of dichloromethane, and added dropwise to the above reaction solution After the dropwise addition was completed, the mixture was stirred at -70°C for 0.5 hour, followed by TLC until the raw material was completely converted. The solvent was removed by rotary evaporation, and the crude product was separated by silica gel column chromatography (petroleum ether / ethyl acetate=12:1, v / v)...
Embodiment 3
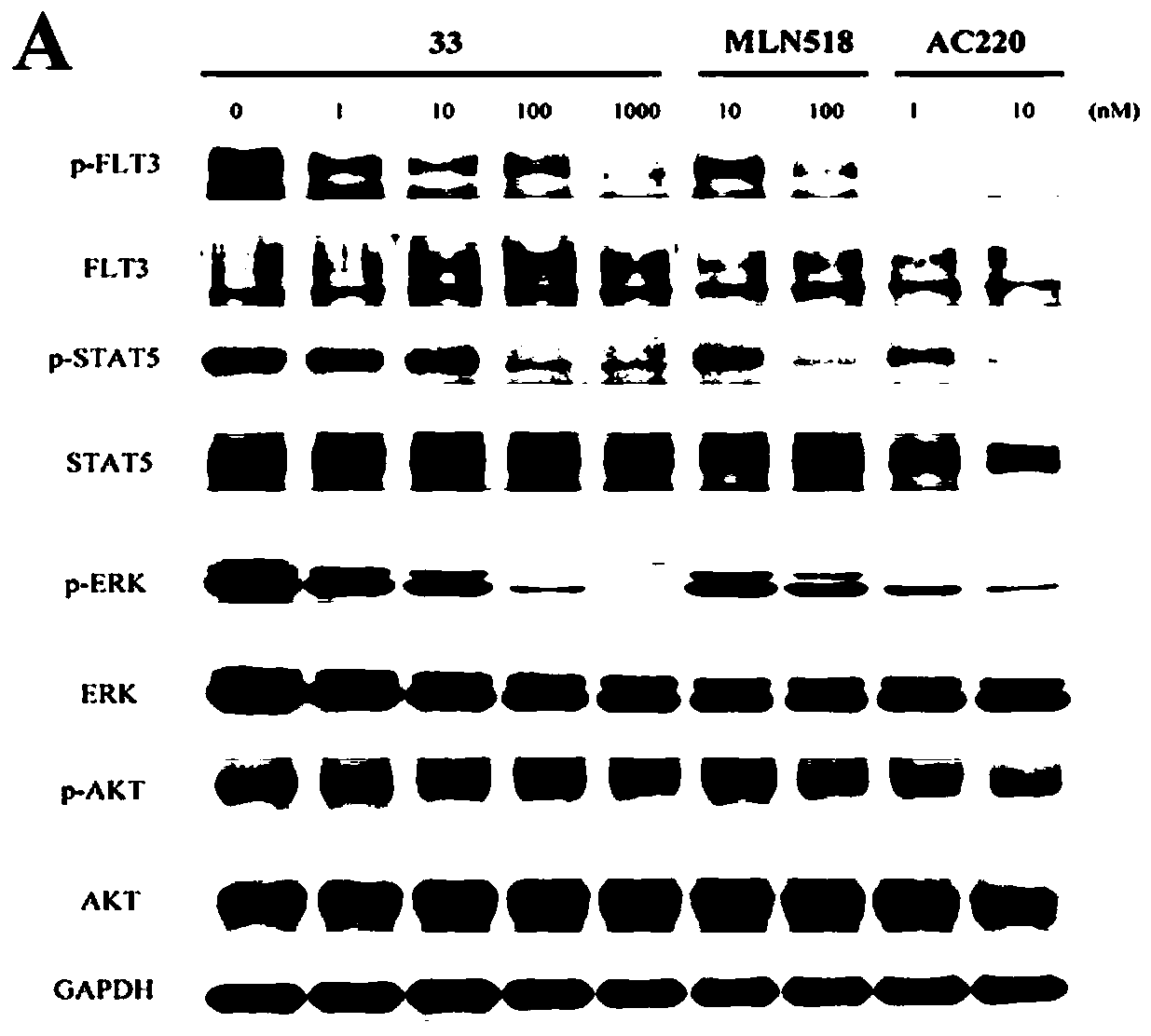
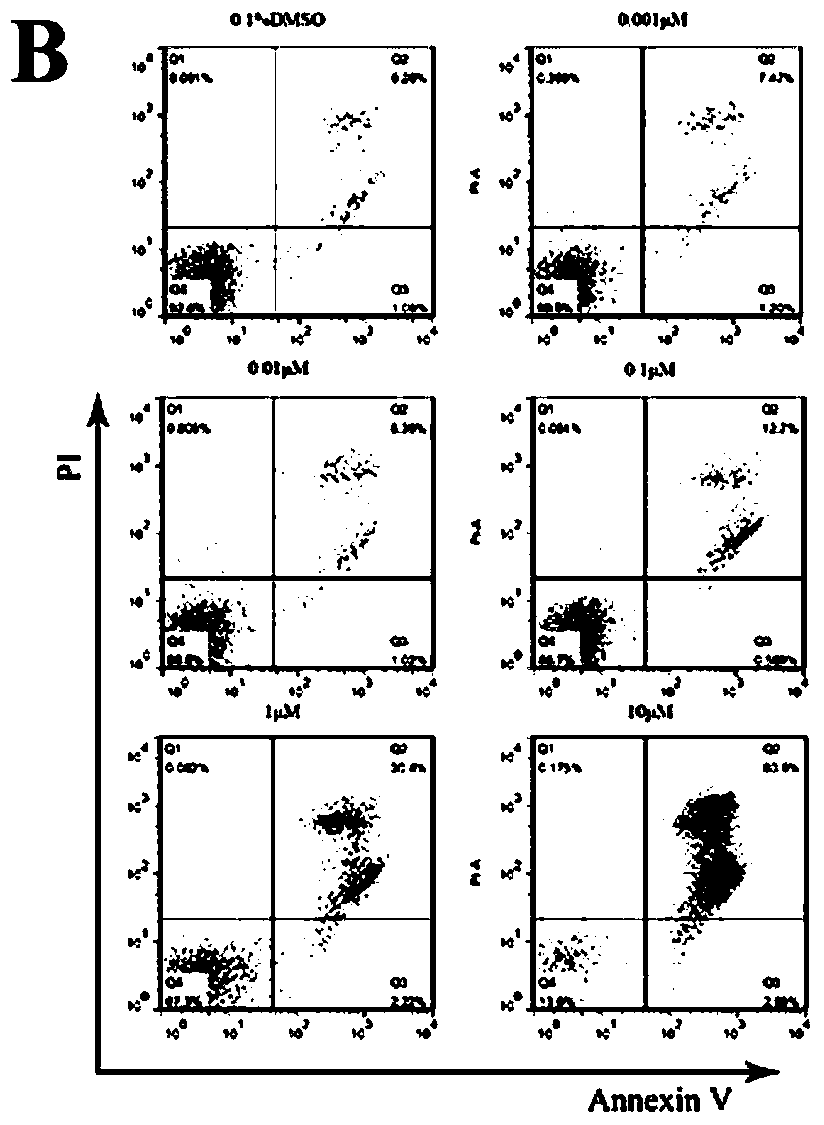
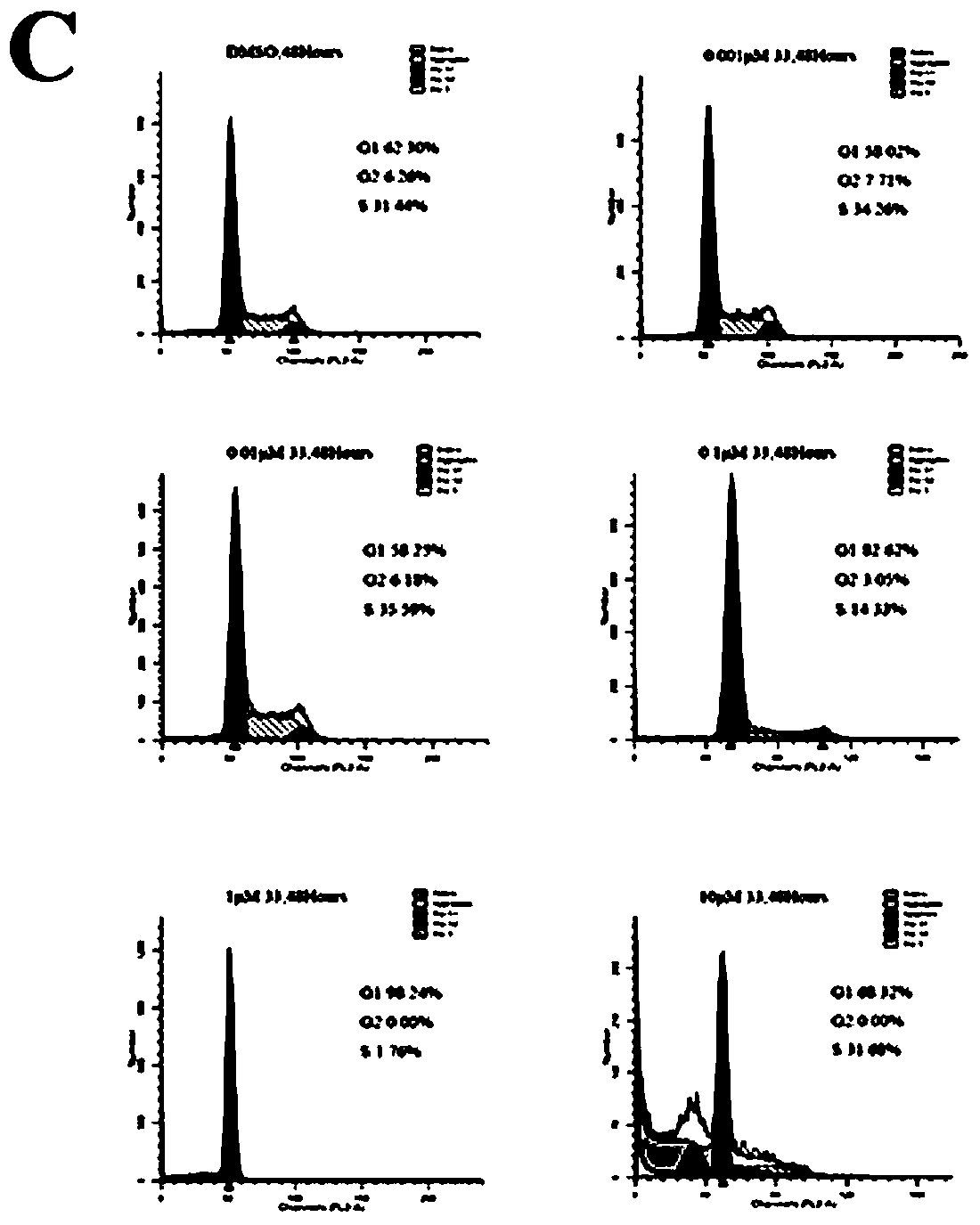
[0250] Biological Activity Test Section
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com