Production method of compound having n,n-bis(2-hydroxy-3-chloropropyl)amino group
A manufacturing method, the technology of chloropropyl, applied in the preparation of organic compounds, the preparation of amino hydroxyl compounds, organic chemical methods, etc., can solve the problems that the production efficiency and safety manufacturing methods have not been established, so as to avoid the risk of loss of control and improve productivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Addition reaction:
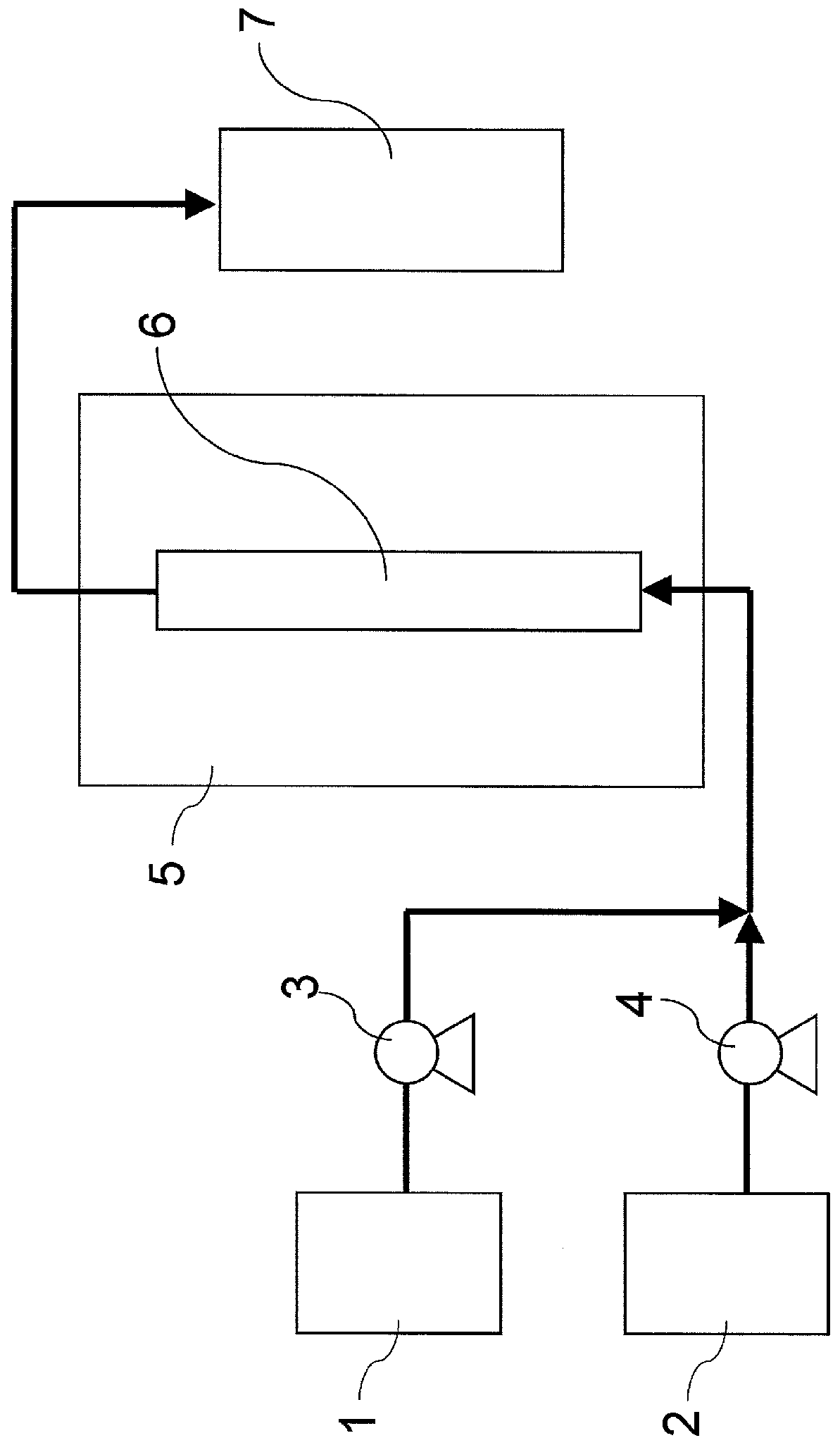
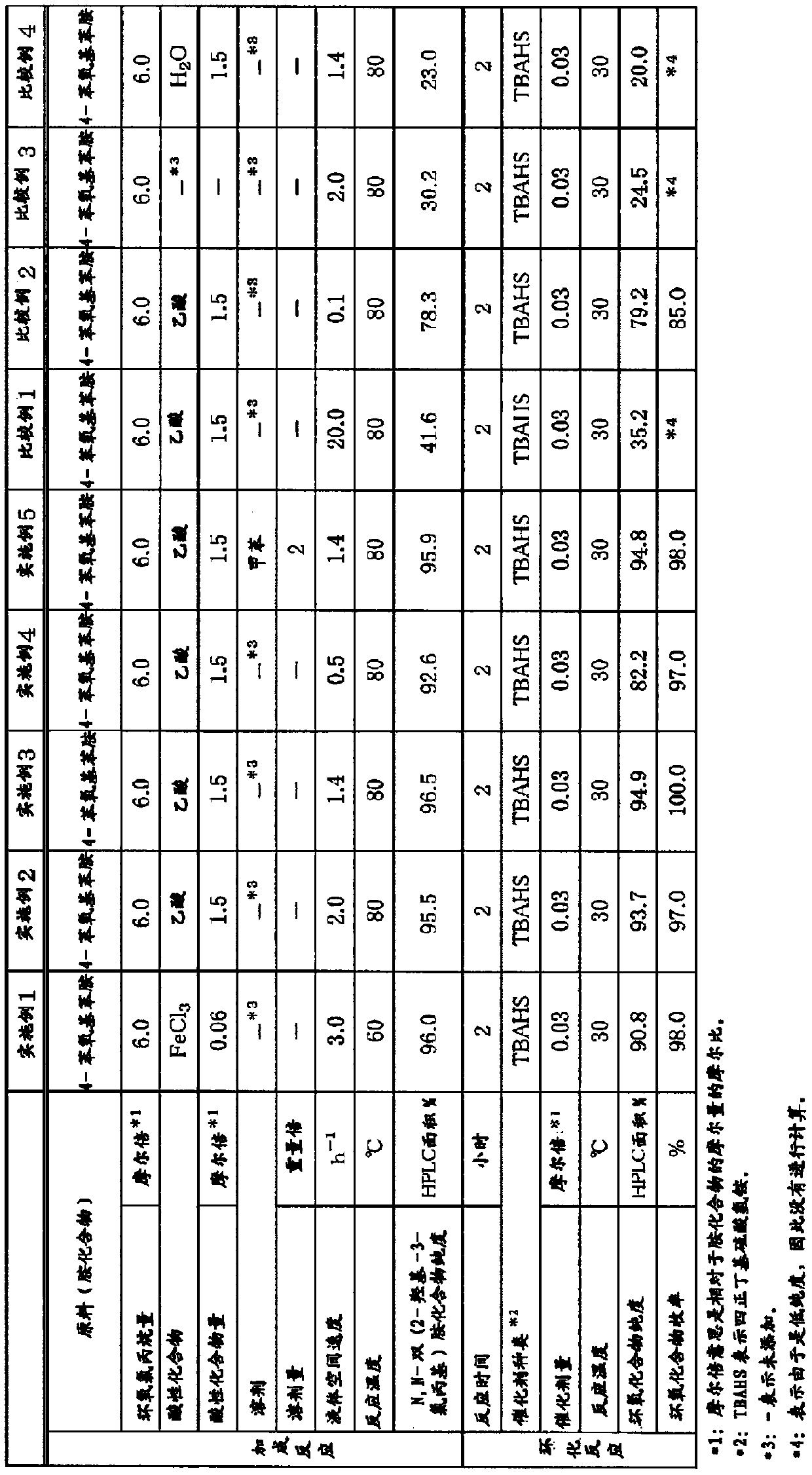
[0094] use figure 1 In the shown device, the 25% by weight (4-phenoxyaniline) / epoxychlorohydrin solution (4-phenoxyaniline:epoxychlorohydrin=1:6 (mole ratio)), and the 11% by weight (iron chloride (III)) / isopropanol solution as an acidic compound solution are fed to a constant temperature of 60° C. at a feed rate of 1.10 g / min. and 0.14 g / min. 5 / 8-inch SUS304 tubular reactor in the tank (inner diameter: 13.4mm, length: 400mm, space volume: 22ml (filled with φ3mm alumina balls)) (the liquid space velocity in the reaction tube of the reaction liquid at this time is 3.0 h -1 ). In addition, the supply amount of iron (III) chloride was 0.06 mole times with respect to the amine compound supplied to the tubular reactor. 150 g of an addition reaction liquid discharged from the outlet of the reactor was obtained. The purity analysis of 4-phenoxy-N,N-bis(2-hydroxy-3-chloropropyl)aniline in the obtained reaction liquid was 96.0% (HPLC area %).
[0095] ...
Embodiment 2
[0099] Addition reaction:
[0100] As an acidic compound solution, instead of the 11% by weight (iron chloride (III)) / isopropanol solution in Example 1, acetic acid was sent to the 80°C constant temperature tank at a rate of 0.13g / min. Addition reaction was carried out in the same manner as in Example 1, except that a 5 / 8-inch SUS304 tubular reactor (inner diameter: 13.4 mm, length: 600 mm, space volume: 33 ml (filled with φ3 mm alumina balls)) was used. The liquid space velocity in the reaction tube of the reaction solution at this time is 2.0h -1. In addition, the supply amount of acetic acid was 1.5 mole times with respect to the amine compound supplied to the tubular reactor. Analysis of the purity of 4-phenoxy-N,N-bis(2-hydroxy-3-chloropropyl)aniline in the obtained reaction liquid revealed a purity of 95.5% (HPLC area %).
[0101] Cyclization reaction:
[0102] The cyclization reaction was carried out in the same manner as in Example 1, except that 164 g (5.0 mole ti...
Embodiment 3
[0105] Addition reaction:
[0106] In Example 2, a 5 / 8-inch SUS304 tubular reactor (inner diameter: 13.4 mm, length: 400 mm, space volume 48 ml (manufactured by Shibata Chemical Co., Ltd., filled with SUS316L Helipack No. 1)) was used, except that , Addition reaction was carried out in the same manner as in Example 2. The liquid space velocity in the reaction tube of the reaction solution at this time is 1.4h -1 . The purity analysis of 4-phenoxy-N,N-bis(2-hydroxy-3-chloropropyl)aniline in the obtained reaction liquid was 96.5% (HPLC area %).
[0107] Cyclization reaction:
[0108] Cyclization reaction was carried out in the same manner as in Example 2 using the reaction solution obtained by the above addition reaction.
[0109] 53.9 g of 4-phenoxy-N,N-diglycidylaniline was obtained by this cyclization reaction (weight yield (4-phenoxyaniline basis): 100%). The chemical purity of the obtained 4-phenoxy-N,N-diglycidylaniline was 94.9% (HPLC area %).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com