Lactic acid bacterium having purine body uptake ability, and use thereof
A technology of lactic acid bacteria and purine, applied in the field of lactic acid bacteria, to achieve the effect of reducing the level of serum uric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
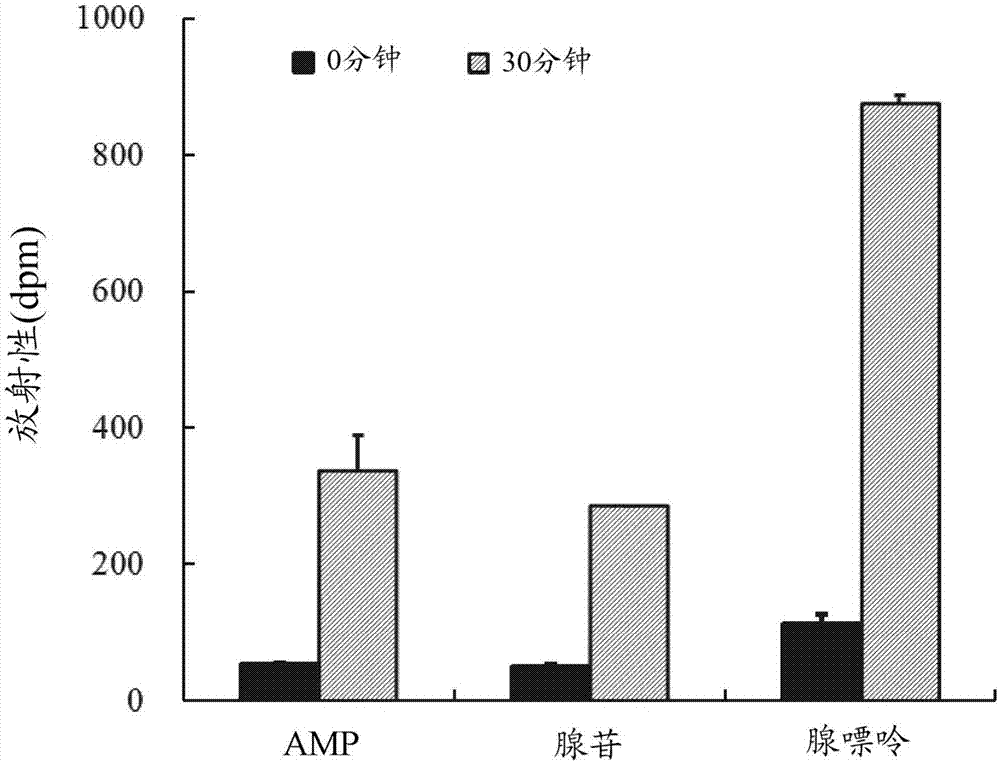
[0086] [Example 1] Test for evaluating purine uptake ability
[0087] In this example, the purine uptake ability of the Lactobacillus gasseri OLL2959 strain was evaluated using purine labeled with a radioisotope (RI).
[0088] Lactobacillus gasseri OLL2959 strain was deposited on March 31, 2006 (original deposit date) under the accession number NITE P-224 at the National Institute of Technology Evaluation, Patent Microorganism Deposit Center (NPMD) (#122, 2-5-8 Kazusakamatari, Kisarazu -shi, Chiba 292-0818, Japan), and was subsequently transferred to a deposit under the Budapest Treaty (International Deposit) on November 21, 2007 under the changed accession number NITEBP-224 from the original accession number. Lactobacillus gasseri OLL2959 strain was inoculated into MRS medium (Lactobacillus MRS Broth, Difco Co., Ltd.), and cultured at 37° C. for 16 to 20 hours, the resulting culture (4 to 7×10 8 cfu / ml) are used below.
[0089] To 0.1 mL of minimal medium (DM medium; Table ...
Embodiment 2
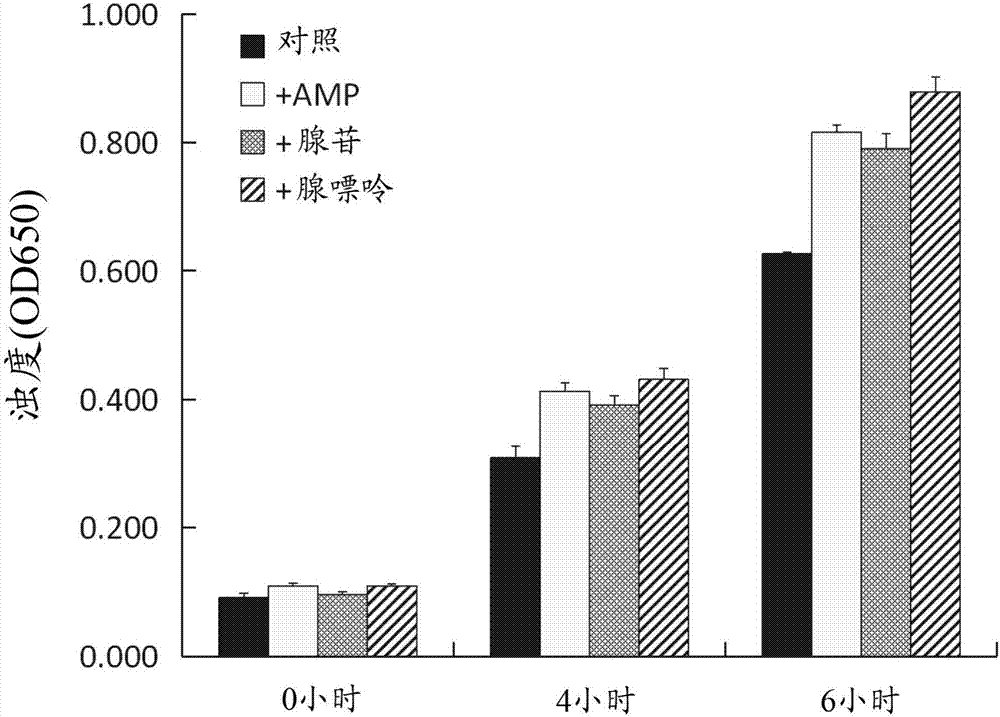
[0097] [Example 2] Test for evaluating proliferative ability in the presence of purine
[0098] In this example, the Bacillus gasseri OLL2959 strain was cultured in the presence of purine, and its proliferative ability in the presence of purine was evaluated.
[0099] To 1 mL of DM medium (Table 1), adenosine monophosphate (AMP), adenosine, or adenine was added as purine to a final concentration of 400 μM, and then the culture of Lactobacillus gasseri OLL2959 strain prepared in Example 1 was With 4wt% (0.04mL:1.6 to 2.8x 10 7 cfu) and cultured anaerobically at 37°C. After 0 hour, 4 hours and 6 hours from the start of this culture, the turbidity (absorbance at 650 nm) of the medium was measured. As a control, the Lactobacillus gasseri OLL2959 strain was cultured in the same manner except that purine was not added to the minimal medium, and the turbidity of the medium was measured. The results are shown in figure 2 middle.
[0100] The results showed that in the presence o...
Embodiment 3
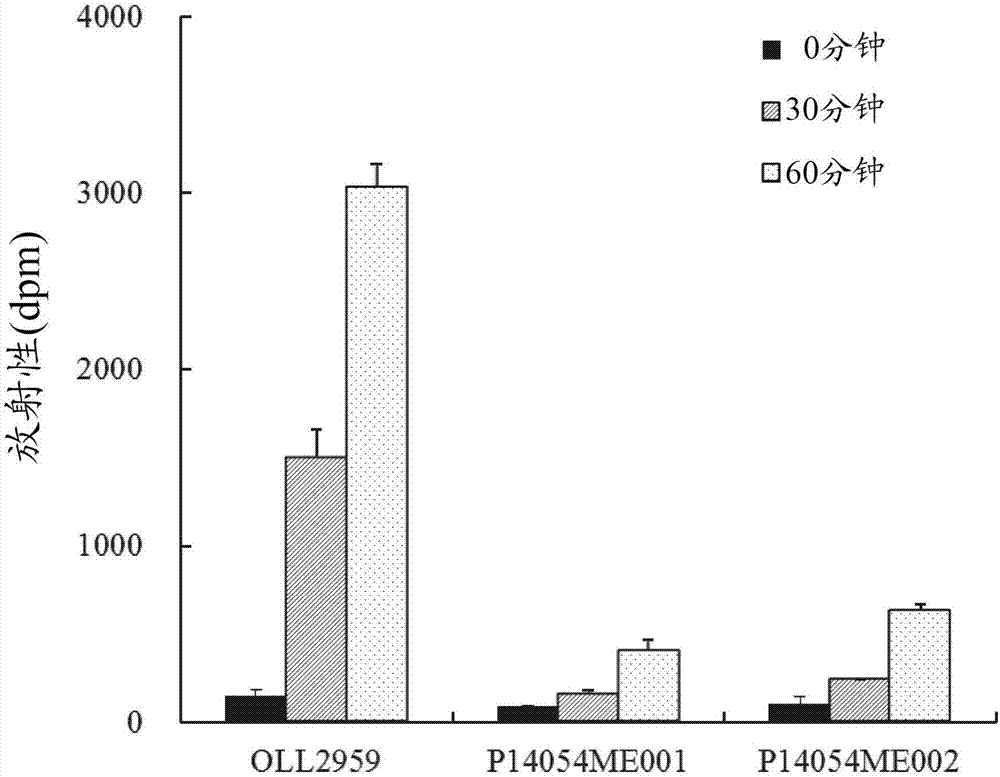
[0101] [Example 3] Comparative test of adenine uptake ability and proliferative ability in the presence of adenine
[0102] In this example, the Lactobacillus gasseri OLL2959 strain and other Lactobacillus gasseri strains were cultured in the presence of adenine, and the adenine uptake ability and proliferation ability in the presence of adenine of the respective strains were compared.
[0103] Lactobacillus gasseri P14054ME001 strain and P14054ME002 strain were used as other Lactobacillus gasseri strains. When culturing Lactobacillus gasseri P14054ME001 strain and P14054ME002 strain in purine-free MRS medium (Lactobacillus MRS broth, Difco, Co., Ltd.) for 20 hours, the proliferation ability of each strain was comparable to that of milk gasseri Bacillus OLL2959 strain was comparable (Table 2).
[0104] [Table 2]
[0105]
[0106] The evaluation test for adenine uptake ability was performed as described in Example 1, except that only adenine ( 14 C-adenine) as a radioisot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com