Pillararene type compound as well as preparation method, pharmaceutical composition and application thereof
A technology of complexes and pillar aromatics, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: Preparation and characterization of CP6A / nitrogen mustard complex
[0097] Preparation and characterization of CP6A / nitrogen mustard complex:
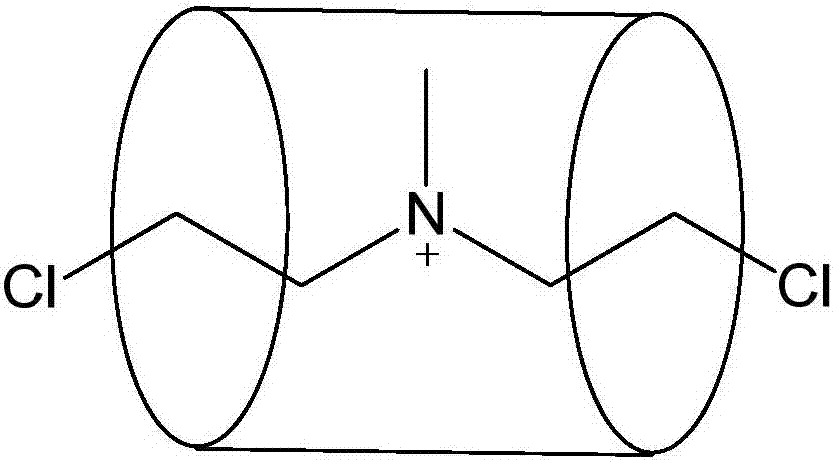
[0098] Accurately weigh 20mg nitrogen mustard (purchased from Beijing Yinuokai Co., Ltd.) (0.105mmol) and 171mg CP6A (M is ammonium ion, the preparation method can refer to J.Am.Chem.SOC.2012,134,13248-13251) (0.105mmol) was mixed and dissolved in 5mL water until it was fully and uniformly mixed, and then the solution of the mixture was vacuum freeze-dried within 10 minutes to obtain a nitrogen mustard / CP6A complex. Composite 1 H-NMR and CP6A alone and nitrogen mustard alone in D 2 O's 1 H-NMR such as Figure 4 , the chemical shift values of the nitrogen mustard in the complex are shown in Table 1 below:
[0099] Table 1
[0100]
[0101] From Table 1, Figure 4 It can be clearly seen that when the nitrogen mustard drug is included by CP6A, its chemical shift value changes significantly, and the H a 、H ...
Embodiment 2
[0102] Example 2: Preparation and Characterization of CP6A / Oxaliplatin Complex
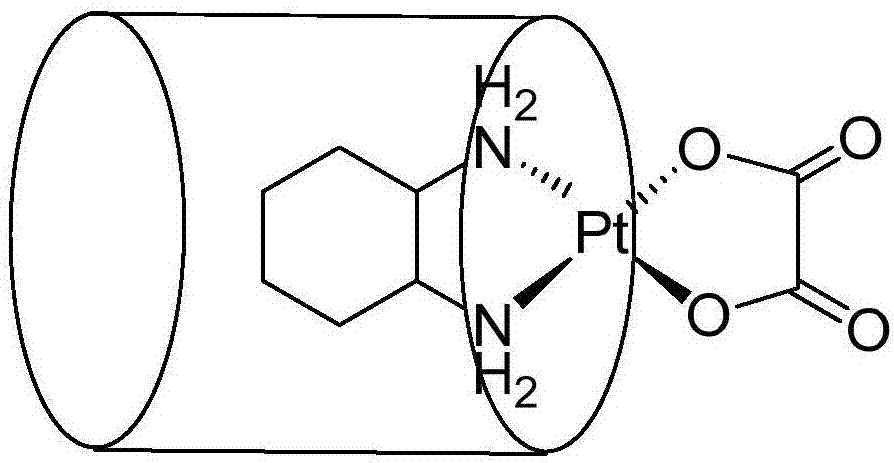
[0103] Accurately weigh 20mg of oxaliplatin (0.05mmol) (purchased from Beijing Yinuokai Co., Ltd.) and 82mg of CP6A (0.05mmol) and mix them in 10mL of water. The drug is completely dissolved and uniformly mixed, and the mixed solution is freeze-dried under vacuum to obtain the CP6A / oxaliplatin complex. Composite 1 H-NMR and individual subject CP6A and individual oxaliplatin drug in D 2 O's 1 H-NMR such as Figure 5 , the chemical shift values of oxaliplatin in the complex are shown in Table 2 below:
[0104] Table 2
[0105]
[0106]
[0107] From Table 2, Figure 5 It can be clearly seen that after oxaliplatin and CP6A form a complex, the chemical shift value changes significantly, and the H a,a’ 、H b,b’ and H c The proton peak broadens significantly, nearly disappears, and moves to high fields. The change value of its chemical shift is shown in Table 2, which shows that the c...
Embodiment 3
[0109] Example 3: Evaluation of the inhibitory effect of CP6A / nitrogen mustard complex on tumor cells
[0110] 1. Experimental samples
[0111] CP6A / nitrogen mustard complex: prepared in Example 1.
[0112] Human breast cancer cell MCF-7 and liver cancer cell HepG2: both provided by Peking Union Medical College Cell Bank.
[0113] 2. Experimental method
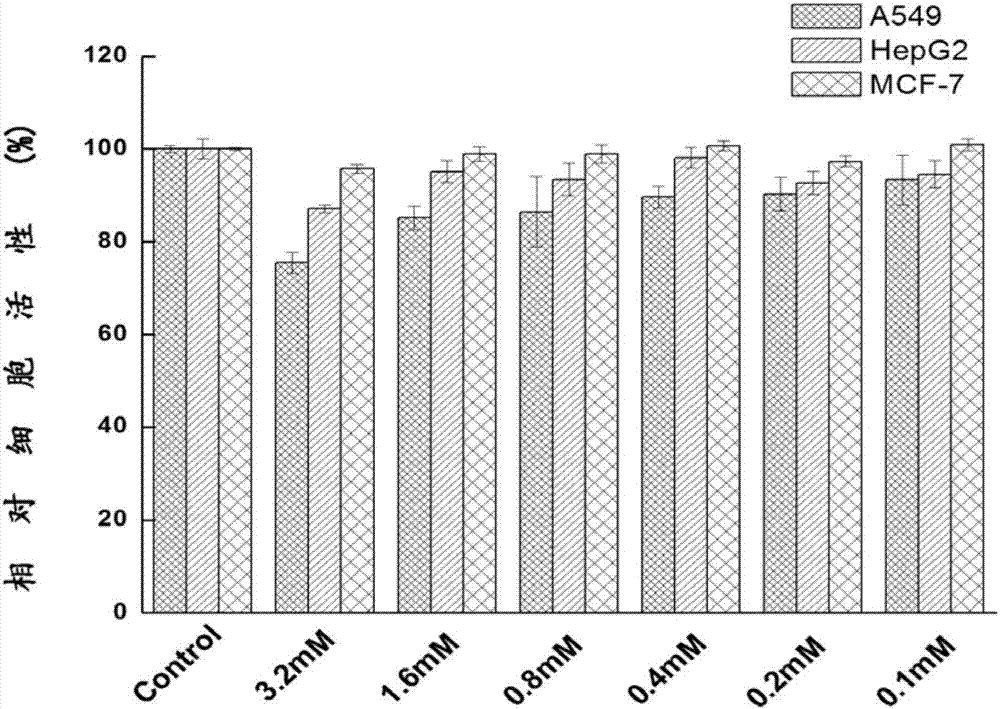
[0114]MTT method was used to evaluate the inhibitory effect of CP6A / nitrogen mustard complex on human breast cancer cell MCF-7 and liver cancer cell HepG2. Nitrogen mustard alone was used as the control group, and its toxicity was measured 48 hours after administration.
[0115] For each type of cell, collect logarithmic growth cells (MCF-7, HepG-2 cells), adjust the concentration of the cell suspension, inoculate the cell suspension in a 96-well plate, and plate to adjust the density of the cells to be tested to about 10,000 / well, 100 μL cell suspension per well, in 5% CO 2 , incubate at a constant temperature of 37°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com