Synthesis method of tert butyl-7-(hydroxymethyl)-7, 8-dihydrogen-4H-pyrazolo diazepine-5(6H)-formyl ester
A technology of a diazepine and a synthetic method, applied in the field of synthesis of tert-butyl-7-hydroxymethyl-7,8-dihydro 4H pyrazolodiazepine 5(6H) formate, reaches Reasonable reaction process design and the effect of saving synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
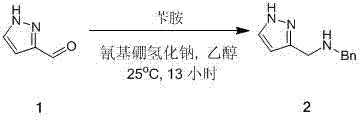
[0009] Synthesis of N-((1H-pyrazol-3-yl)methyl)-1-phenylmethylamine
[0010]
[0011] 200 g of 1 was added to 1.5 liters of absolute ethanol solution, 25 o Add 223 grams of benzylamine and 5 grams of acetic acid to C, and stir at 25°C for 2 hours, then add 196 grams of sodium cyanoborohydride in batches at 25°C, and continue to stir the reaction system for 13 hours. The reaction system was quenched with 200 ml of saturated ammonium chloride solution, concentrated in vacuo to remove the organic phase, extracted with dichloromethane (500 ml*5), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 400 g of crude compound 2 yellow Oil.
Embodiment 2
[0013] Synthesis of ethyl 5-benzyl-5,6,7,8-tetrahydro-4H-pyrazolo[1,5-a][1,4]diazepine-7-carboxylate
[0014]
[0015] Dissolve 449 g of compound 2 in 2 liters of ethanol solution, add 463 g of compound 3 at 10°C, stir at 25°C for 12 hours, and then stir at 70°C for 12 hours. After the reaction, the reaction solution was cooled to 20° C., concentrated in vacuo to obtain a crude product, which was then purified by a silica gel column and recrystallized from 3 liters of ethyl acetate to obtain a white compound 4 with a two-step yield of 20%.
Embodiment 3
[0017] Synthesis of ethyl 5,6,7,8-tetrahydro-4H-pyrazolo[1,5-a][1,4]diazepine-7-carboxylate
[0018]
[0019] Dissolve 80 g of compound 4 in 0.8 liter of absolute ethanol solution, add 20 g of 10% palladium carbon, and replace with hydrogen three times. The reaction was stirred at 50°C for 10 hours in the presence of 50 Psi hydrogen. After the reaction, the reaction system was filtered through diatomaceous earth to obtain an ethanol solution of Compound 5. used directly in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com