Application of scutellarin biotin-labeled probe and related PKM2 kinase inhibitor
A biotin-labeled, scutellarin aglycone technology, which can be used in drug combination, bulk chemical production, organic chemistry, etc., can solve the problem of low activity of inhibiting PKM2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
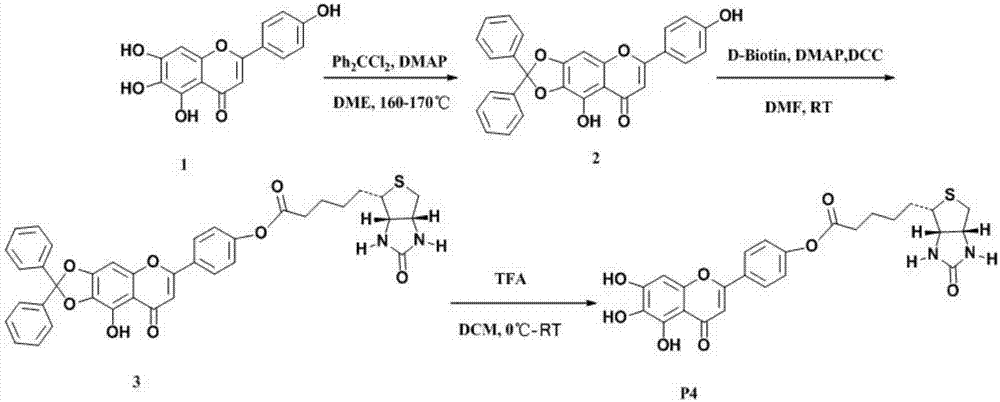
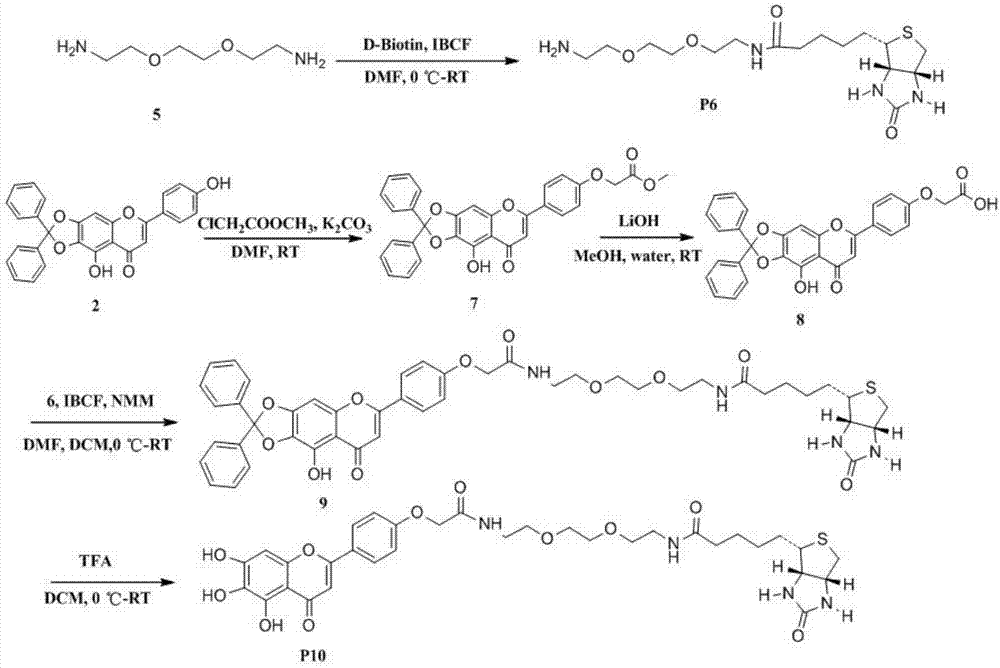
[0037] Preparation principle of scutellarin biotin-labeled probe: After the hydrogen atoms on the hydroxyl groups of scutellarin aglycone 5, 6, 7, and 4' are protected by dibenzocarbone, scutellarin aglycone is directly or indirectly combined with biotin connected, and finally the protective group was removed under the action of TFA to obtain two different scutellarin biotin-labeled probes. Such as figure 1 and figure 2 shown.
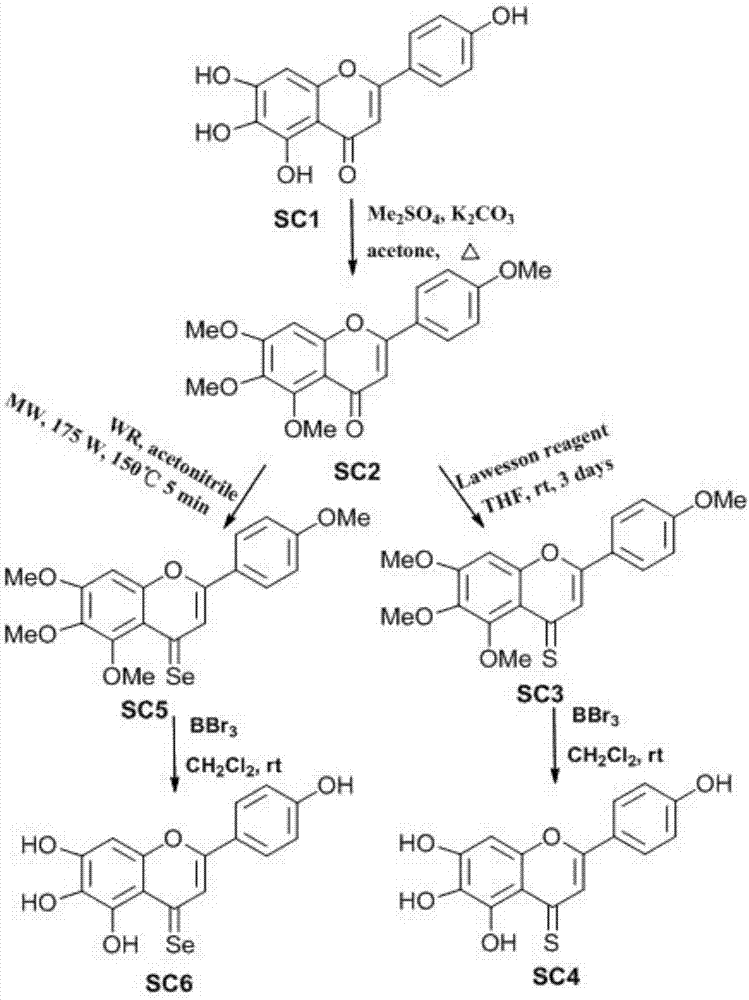
[0038] Preparation principle of scutellarin aglycon derivatives: After the hydrogen atoms on the hydroxyl groups of scutellarin aglycone 5, 6, 7, and 4' are replaced by methyl groups, the oxygen at the 3-position is replaced by Lawson's reagent and WR reagent respectively For intermediates containing sulfur and selenium, finally in BBr 3 Under the action of removing the protecting group methyl, two derivatives different from scutellarin aglycone are obtained. Such as image 3 shown.
Embodiment 1
[0041] Preparation of ketal protected scutellarin aglycone (2):
[0042]
[0043] Take the dried scutellarin aglycone (1) (0.5g, 1.75mmol) and place it in a dry 100mL two-necked bottle, add 10mL DME to dissolve it, add DMAP (0.25g, 1.75mmol), add dichlorodimethoxylate under stirring at room temperature Benzene methane (0.5mL, 1.75mmol) was reacted at 180°C under reflux for 2h, the reaction was complete as monitored by TLC, the solvent was removed by rotary evaporation under reduced pressure, and the residue was separated on silica gel to obtain light brown powdery solid (2), 0.34g, The yield is 62%. 1 H-NMR (400MHz, DMSO-d6) δ (ppm): 7.92 (d, J = 8.8Hz, 2H), 7.56-7.53 (m, 4H), 7.46-7.44 (m, 6H), 7.04 (s, 1H ),6.90(d,J=8.8Hz,2H),6.83(s,1H).EI-MS(m / z)[M+H] + 451.11.
[0044] Preparation of scutellarin aglycon-4'-biotin (3):
[0045]
[0046] Take the ketal-protected scutellarin aglycon (2) (0.4g, 0.89mmol) in a 100mL eggplant-shaped bottle, add DMAP (0.054g, 0.44mmol) ...
Embodiment 2
[0066] The scutellarin aglycon derivatives are self-prepared in the present invention, and the preparation method is as follows:
[0067] Preparation of compound SC2:
[0068]
[0069] Take scutellarin aglycone (1g, 3.49mml) and K 2 CO 3 (2.41g, 17.47mml) in 15mL acetone solution, oil bath to reflux, slowly add 1.3mL dropwise, after addition, reflux reaction for 2h, TLC monitors that the reaction is complete, extract with ethyl acetate (300mL×2), and the organic layer is water (300mL×2) washing, anhydrous MgSO 4 After drying, ethyl acetate was recovered under reduced pressure to dryness, and silica gel column chromatography gave light yellow powdery solid SC2 (0.96 g), with a yield of 85%. 1 H-NMR (400MHz, DMSO-d6) δ (ppm): 7.92 (d, J = 8.8Hz, 2H), 6.90 (d, J = 8.8Hz, 2H), 6.83 (s.1H), 6.30 (s. 1H),3.95(s.12H).EI-MS(m / z)[M+H] + 343.34.
[0070] Preparation of compound SC3:
[0071]
[0072] Take compound SC2 (0.5g, 1.46mml) and Lowe's reagent (0.59g, 1.46mml) in 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com