Preparation method of 6-Chloromethylmorphanthridine
A technology of chloromethylmorphidine and phenylmethyl, which is applied in the field of preparation of 6-chloromethylmorphidine, which can solve the problems of large amount of waste water, instability, and serious environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
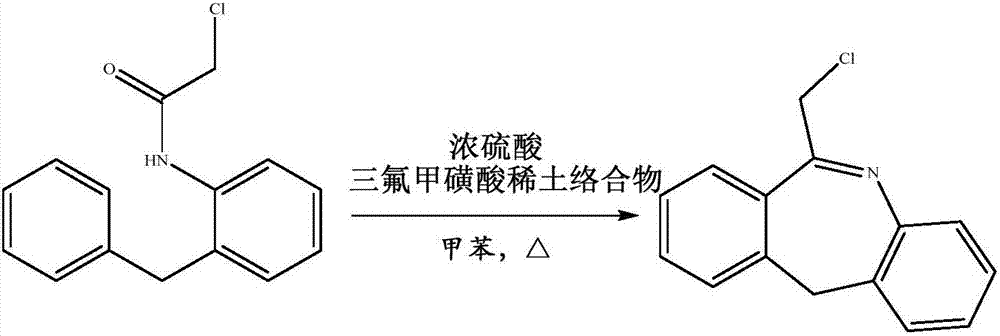
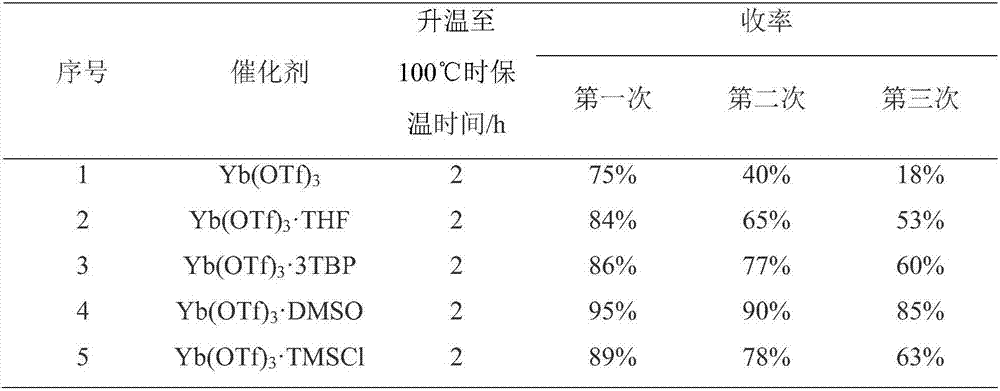
[0031] 26.0g (0.10mol) of N-[2-(phenylmethyl)phenyl]-2-chloroacetamide and 200ml of toluene were added to a 500ml four-neck flask, 2.0g (0.02mol) of concentrated sulfuric acid was added, stirred, and 0.62 g (0.001mol) of ytterbium trifluoromethanesulfonate and 1ml of DMSO, heated up to 100°C, and kept at this temperature for 2 hours. During the reaction, the water was removed. At the end of the heat preservation, about 75ml of toluene was distilled out, the temperature was lowered to 10°C, heat preservation was carried out for 1 hour, suction filtered, and dried at 50°C for 8 hours to obtain 23g of 6-chloromethylmorpholidine with a purity of 99.3% and a yield of 95.1%.
[0032] Mix the suction-filtered mother liquor and the distilled toluene, then add 26.0g of N-[2-(phenylmethyl)phenyl]-2-chloroacetamide, operate according to the above method, apply the mother liquor once to get 6-chloro 21.9 g of methylmorpholidine, with a purity of 98.7%, and a yield of 90.8%.
[0033] The ...
Embodiment 2
[0035] 26.0g (0.10mol) of N-[2-(phenylmethyl)phenyl]-2-chloroacetamide and 180ml of toluene were added to a 500ml four-neck flask, 1.0g (0.01mol) of concentrated sulfuric acid was added, stirred, and 2.93 g (0.005mol) lanthanum trifluoromethanesulfonate and 1ml DMSO, heat up to 90°C, keep warm for 2.5 hours, remove water during the reaction. At the end of the heat preservation, about 75ml of toluene was distilled out, then the temperature was lowered to 5°C, heat preservation was carried out for 2 hours, suction filtered, and dried at 40°C for 10 hours to obtain 23.1g of 6-chloromethylmorpholidine with a purity of 99.1% and a yield of 95.8%.
[0036] Mix the suction-filtered mother liquor and the distilled toluene, then add 26.0g of N-[2-(phenylmethyl)phenyl]-2-chloroacetamide, operate according to the above method, apply the mother liquor once to get 6-chloro 22 g of methyl morpholidine, with a purity of 98.9% and a yield of 91%.
Embodiment 3
[0038] Add 26.0g (0.10mol) of N-[2-(phenylmethyl)phenyl]-2-chloroacetamide and 190ml of toluene into a 500ml four-necked flask, add 3.0g (0.03mol) of concentrated sulfuric acid, stir, and add 0.98 g (0.002mol) scandium trifluoromethanesulfonate and 1ml DMSO, heat up to 95°C, keep warm for 3 hours, remove water during the reaction. At the end of the heat preservation, about 75ml of toluene was distilled out, the temperature was lowered to 2°C, heat preservation was carried out for 1 hour, suction filtered, and dried at 45°C for 9 hours to obtain 23g of 6-chloromethylmorphoprenidine with a purity of 98.7% and a yield of 95.3%.
[0039] Mix the suction-filtered mother liquor and the distilled toluene, then add 26.0g of N-[2-(phenylmethyl)phenyl]-2-chloroacetamide, operate according to the above method, apply the mother liquor once to get 6-chloro 21.8 g of methylmorpholidine, with a purity of 98.5%, and a yield of 90.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com