Preparation method of substituted cyclopentadienyl metallocene compound
A metallocene compound and cyclopentadienyl technology, which is applied in the field of preparation of metallocene compounds, can solve problems such as undiscovered, and achieve the effects of easy quantification, good economy, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
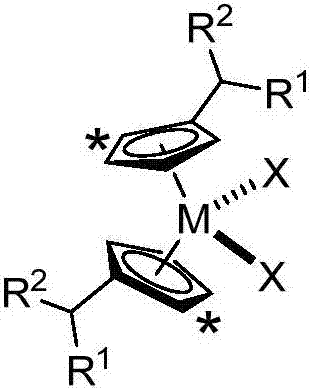
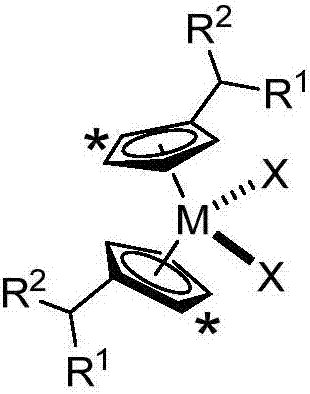
[0038] The preparation method of the substituted cyclopentadienyl metallocene compound, the structure of the prepared metallocene compound is as follows:
[0039]
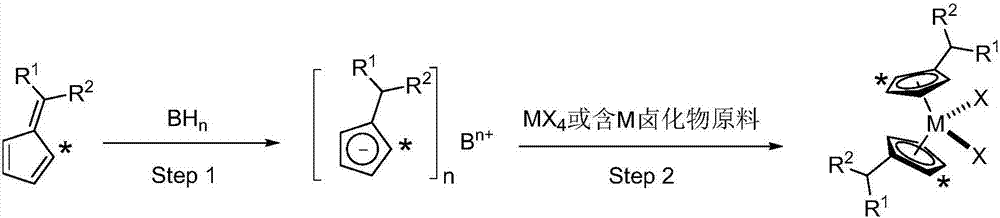
[0040] The preparation process includes the following steps:
[0041] In the first step, the substituted fulvene compound reacts with the hydride of an alkali metal or alkaline earth metal to obtain a substituted cyclopentadienyl salt;
[0042] In the second step, the substituted cyclopentadienyl salt is further reacted with a Group IV halide or a derivative of a Group IV halide to obtain a metallocene compound. The process is as follows:
[0043]
[0044] In the formula: the symbol "*" indicates that the five-membered ring has 0-4 non-hydrogen substituents, and the non-hydrogen substituents are independently of each other preferably containing 1 to 30 carbon atoms containing heteroatom or no heteroatom the alkyl group of the atom;
[0045] R 1 and R 2 are independently selected from hydrogen, heteroatom...
Embodiment 1
[0067] Synthesis of 6,6-Dimethylfulvene
[0068] 500 mL of ethanol was added to the reactor, 16.9 g of sodium metal (0.735 mol) was added in batches, stirred for 2 hours, 38.7 g of acetone (0.666 mol) was added, 50.0 g of cyclopentadiene (0.765 mol) was added dropwise at room temperature, and the mixture was stirred overnight. The reaction was quenched with 100 mL of acetic acid and 500 mL of water, allowed to stand, and the solution was separated. The organic phase was taken as a crude product and distilled to obtain 45.5 g of the target product, with an isolated yield of 65%. 1 HNMR (500MHz, CDCl 3 ): δ6.36-6.38(m, 2H), 6.24-6.26(m, 2H), 1.85(s, 6H).
Embodiment 2
[0070] Synthesis of 3,6,6-Trimethylfulvene
[0071] The 12.6 g o-chlorotoluene (0.1 mol) added in Example 1 was changed to 14.2 g o-chloroanisole (0.1 mol), other conditions remained unchanged, and 47.2 g of the target product was obtained by distillation, and the isolated yield was 51%. 1 H NMR (500MHz, CDCl 3 ): δ6.43-6.48(m, 2H), 6.27(s, 1H), 2.06(s, 3H), 1.85(s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com