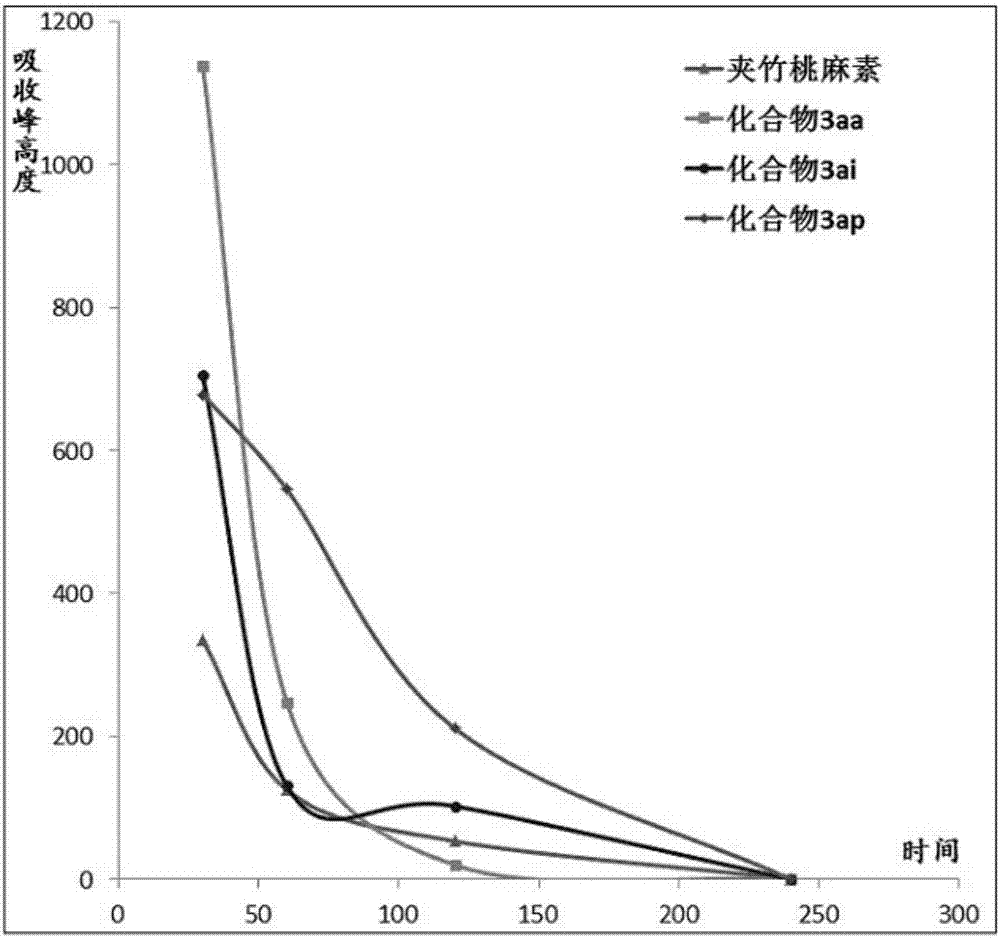
Apocynin water-soluble prodrug, preparation method and pharmaceutical composition thereof, and application of apocynin water-soluble prodrug and pharmaceutical composition thereof
A compound and halogen technology, applied in the design and preparation of prodrugs, can solve the problems of limiting the development and utilization of oral drugs, unsatisfactory bioavailability, and poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: Preparation of N-tert-butoxycarbonyl-L-methionine ester:
[0090]
[0091] Specific steps:
[0092] Weigh apocynin (1.2mmol, 200mg) and dissolve it in dichloromethane (15ml), add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (1.6mmol, 300mg), 1-hydroxybenzotriazole (HOBT) (1.6mmol, 216mg) and N-Boc-L-methionine (1.5mmol, 375mg), stirred at room temperature overnight. After the reaction was complete, add water to quench, extract 3 times with ethyl acetate, combine the organic phases, wash with water and saturated brine successively, anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure, separated and purified on a silica gel column (solvent ratio PE / EA=1:3) to obtain the white solid product 2aa (465 mg), and the yield was 97%.
[0093] The specific characterization data of this product 2aa are:
[0094] 1 H NMR (400MHz, Chloroform-d) δ7.59(s,1H),7.56(d,J=8.1Hz,1H),7.16(d,J=8.0Hz,1H),5.29(d,J=9.5Hz ...
Embodiment 2
[0095] The preparation of embodiment 2.N-BOC-O-benzyl-L-serine ester:
[0096]
[0097] N-Boc-O-benzyl-L-serine (1.5mmol, 443mg) was used as starting material, and the white solid product 2ab (507mg) was prepared by the same method as compound 2aa, with a yield of 95%.
[0098] The specific characterization data of product 2ab are:
[0099] 1 H NMR (400MHz, Chloroform-d) δ7.60(s, 1H), 7.55(dd, J=1.6, 8.2Hz, 1H), 7.39–7.25(m, 5H), 7.13(d, J=8.2Hz, 1H), 5.55(d, J=8.8Hz, 1H), 4.78(d, J=8.9Hz, 1H), 4.64(s, 2H), 4.11(dd, J=9.3, 3.2Hz, 1H), 3.87( dd,J=9.5,3.1Hz,1H),3.82(s,3H),2.61(s,3H),1.48(s,9H); LRMS(ESI)m / z:C 24 h 29 NO 7 ,found 466.1[M+Na] + .
Embodiment 3
[0100] Example 3. Preparation of (S)-2,6-di-tert-butoxycarbonylaminocaproate:
[0101]
[0102] (S)-2,6-Di-tert-butoxycarbonylaminocaproic acid (1.5mmol, 521mg) was used as starting material, and the white solid product 2ac (565mg) was prepared by the same method as compound 2aa, with a yield of 95%.
[0103] The specific characterization data of product 2ac are:
[0104] 1 H NMR (400MHz, Chloroform-d) δ7.60(s, 1H), 7.56(d, J=8.3Hz, 1H), 7.15(d, J=8.1Hz, 1H), 5.23(d, J=7.7Hz ,1H),4.62(dd,J=6.8,41.0Hz,2H),3.87(s,3H),3.17(d,J=5.7Hz,2H),2.61(d,J=4.9Hz,3H),2.12 –1.76(m,3H),1.66–1.50(m,3H),1.47(s,9H),1.44(s,9H); LRMS(ESI)m / z:C 25 h 38 N 2 o 8 ,found 517.1[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com