Application of naringenin nano inhalant in treating acute lung injury
An inhalant and naringenin technology, which is applied in the field of pharmacy, can solve the problems of limited dosage, single route of administration, and poor therapeutic effect, and achieve the goal of solving technical limitations, treating acute lung injury, and inhaling therapeutic effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1. Naringenin phospholipid complex
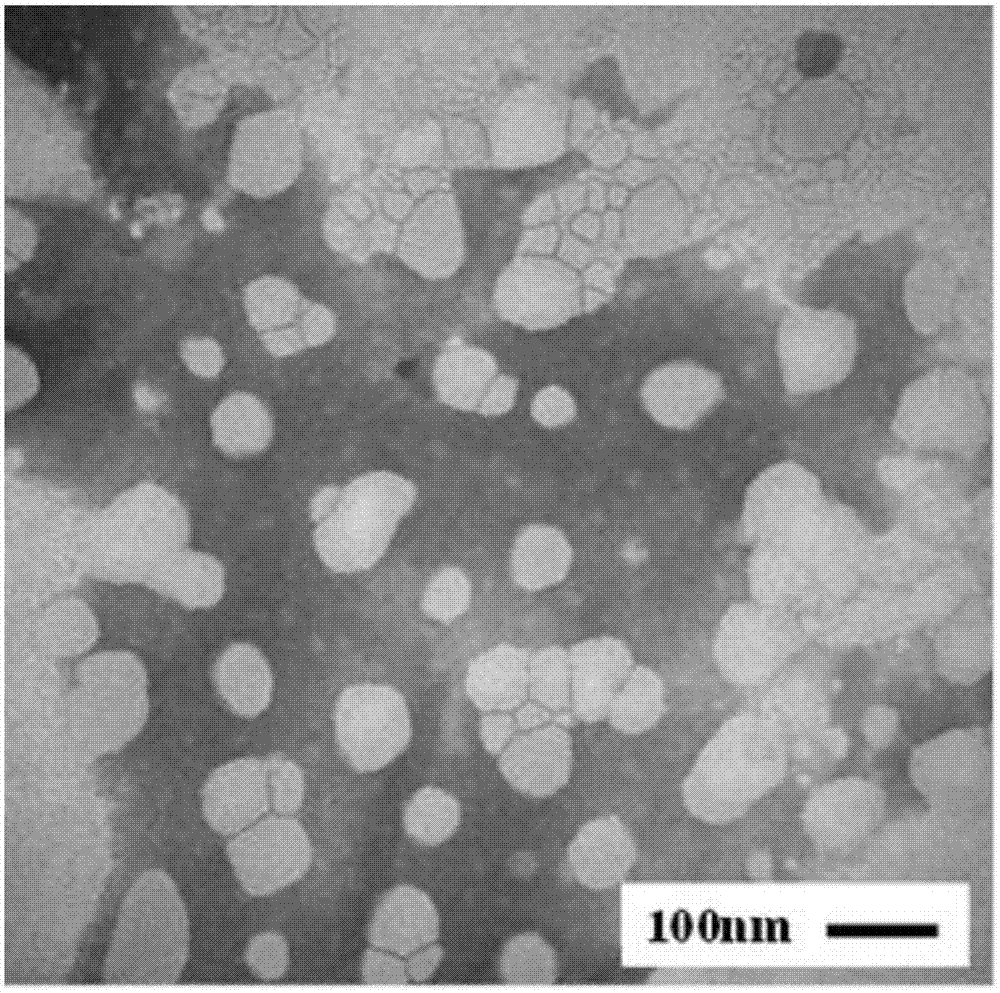
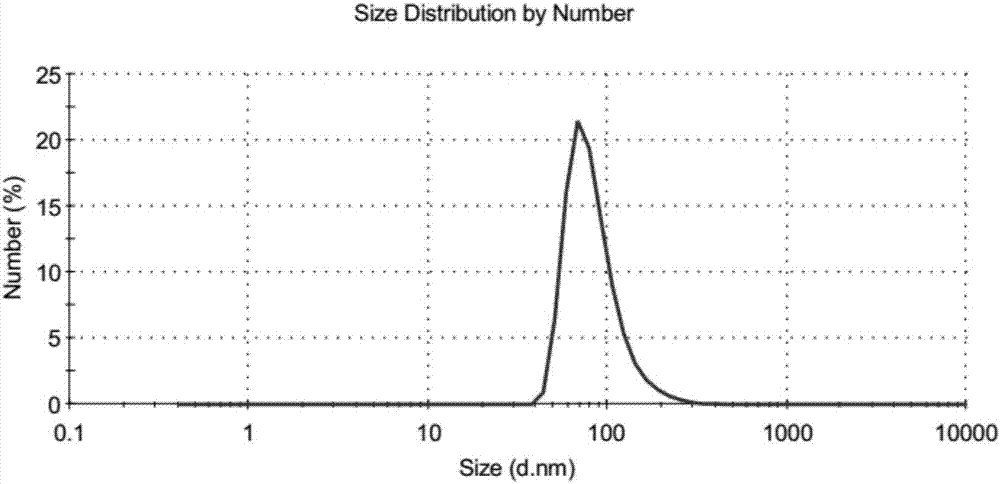
[0048] Take 50 mg of naringenin and 138 mg of dipalmitoyl lecithin and dissolve them together in an appropriate amount of ethanol solvent, sonicate to form a clear solution, and then rotary evaporate in a water bath at 35°C to remove the ethanol solvent to obtain the naringenin-phospholipid complex. The particle size measurement results showed that most of the particles were below 100nm. The distribution of naringenin-phospholipid complexes was observed under a transmission electron microscope, and most of them were spherical ( figure 1 ). The particle size peak value of the naringenin-phospholipid complex measured by a Malvern laser particle size analyzer is 86.82nm ( figure 2 ), PDI is 0.243, and the particle size range is concentrated between 60-90nm.
Embodiment 2
[0049] Embodiment 2. Naringenin solid lipid nanoparticles
[0050] Take 0.1g of naringenin, 2.0g of cetyl palmitate, and 0.4g of caprylic / capric glycerides, heat them in a water bath at 53°C until they melt, and mix them with 1.0g of naringenin as the oil phase. Phospholipid 1.5g, polyoxyethylene 660-12 hydroxystearate 1.8g, add water for injection 100mL, stir and dissolve, heat to 53°C, as the water phase; under the condition of stirring and nitrogen, add the water phase dropwise to the same temperature In the oil phase of the mixture, continue to stir for a certain period of time to form colostrum, ultrasonically probe for 5 minutes, stir at 0-2°C for 2 hours, and filter through 0.22 μm micropores to obtain naringenin solid lipid nanoparticles. Observed under a scanning transmission electron microscope, most of them are particles below 100 nanometers.
Embodiment 3
[0051] Embodiment 3. Naringenin liposomes
[0052] Take 100mg of naringenin, 320mg of soybean lecithin, and 30mg of cholesterol in an eggplant-shaped flask, add 5ml of tetrahydrofuran to dissolve completely, and then evaporate in a water bath at 35°C to remove the organic solvent, and form a thin uniform film on the inner wall of the eggplant-shaped flask; Add 200ml of pH 5.0 phosphate buffer solution with 2.26g of lactose into an eggplant-shaped flask, place it at 37°C and shake at a constant temperature of 100 rpm for 1 hour to fully hydrate it, take it out and ultrasonically disperse it for 10 minutes to obtain naringenin lipid plastid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com