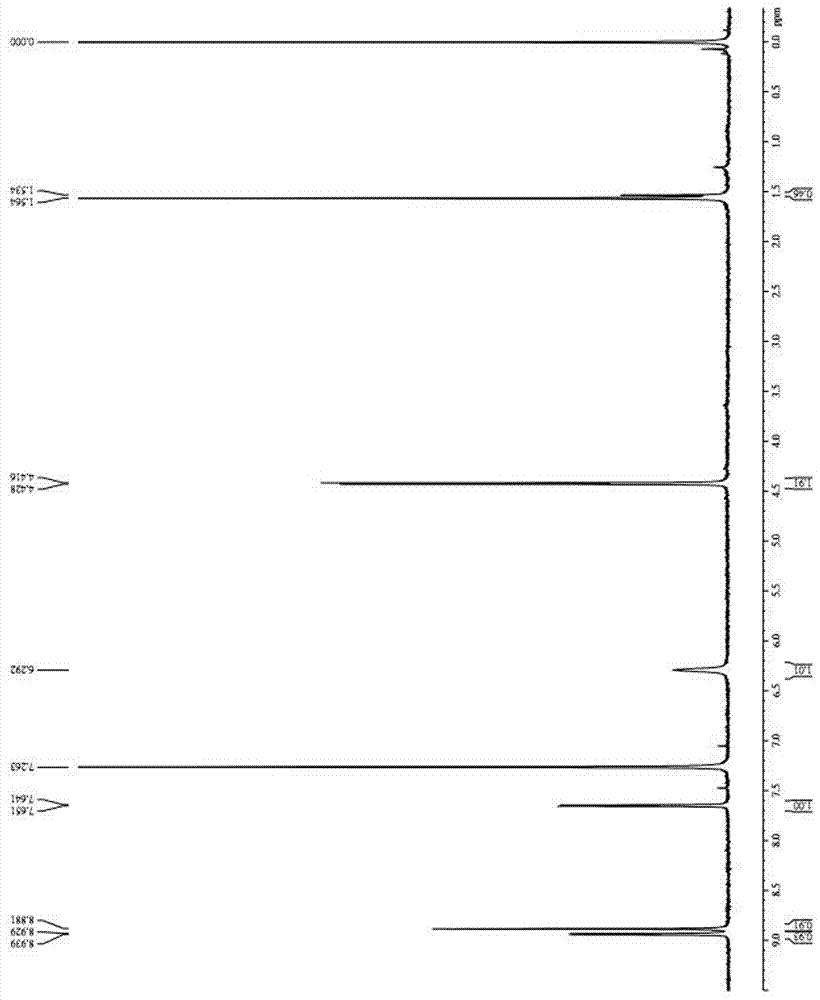
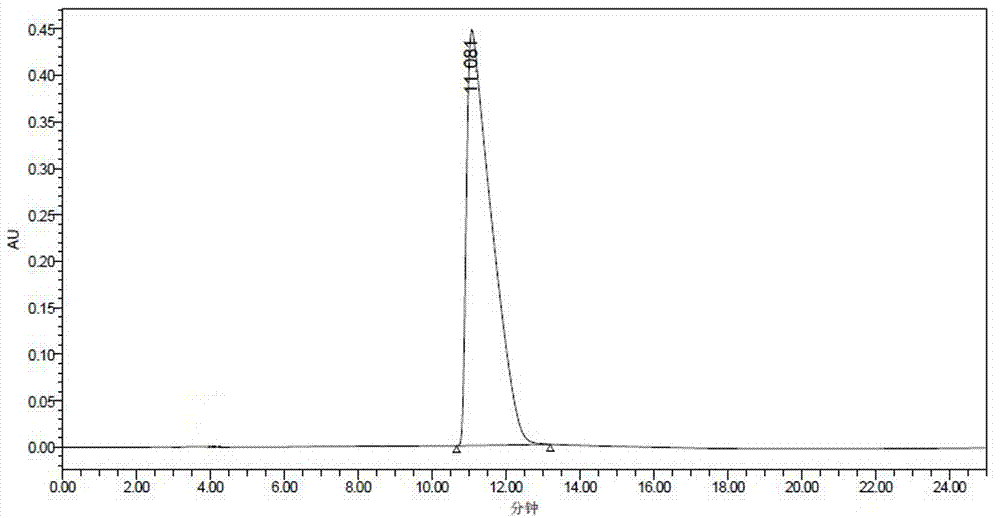
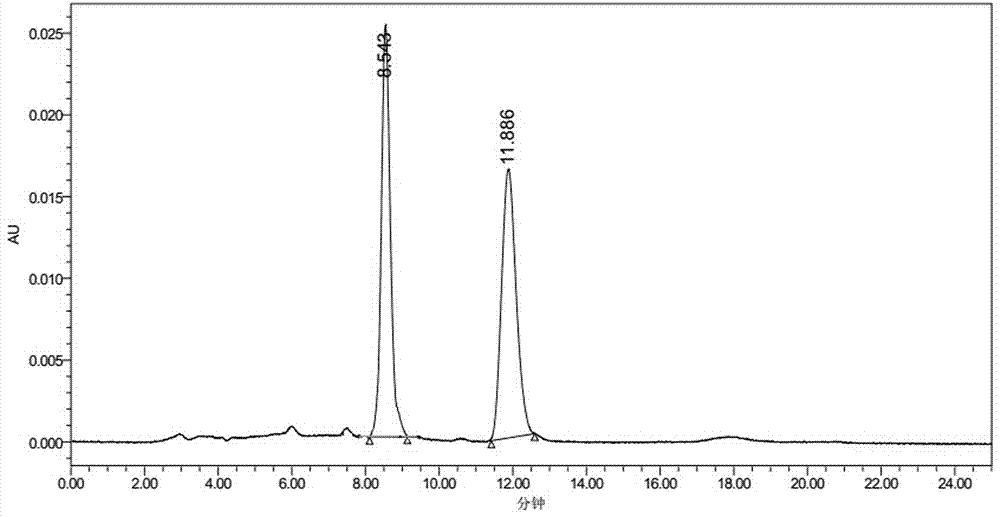
Method for transforming N-cyanomethyl bis(trifluoromethyl) nicotinamide into flonicamide and application thereof
A technology of trifluoromethyl nicotinyl and flonicamid, which is applied in the preparation of pesticides, and converts N-cyanomethyl bis(trifluoromethyl) nicotinamide into flonicamid, which can solve the difficulty of product purification, The problem of high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 A method for converting N-cyanomethylbis(trifluoromethyl)nicotinamide into flonicamid
[0061] A method for converting N-cyanomethylbis(trifluoromethyl)nicotinamide into flonicamid, the synthetic route of which is:
[0062] ;
[0063] Wherein, the transformation reagent is an alkaline aqueous solution, an alkaline alcohol solution or an alkaline alcohol-water mixture;
[0064] The R is -H, -CH 3 、-C 2 h 5 One of them, if higher alcohols are used, the conversion rate will decrease.
[0065] The conversion reagent is an aqueous solution of triethylamine, an alcoholic solution of triethylamine or an alcohol-water mixture of triethylamine;
[0066] Or an aqueous solution of pyridine, an alcohol solution of pyridine or a mixture of alcohol and water of pyridine;
[0067] Or NaOH aqueous solution, NaOH alcohol solution or NaOH alcohol water mixture;
[0068]
[0069] 1) When the conversion reagent is an aqueous solution of triethylamine, the specific con...
Embodiment 2
[0080] Example 2 Application of a method for converting N-cyanomethylbis(trifluoromethyl)nicotinamide into flonicamid
[0081] An application of a method for converting N-cyanomethylbis(trifluoromethyl)nicotinamide into flonicamid, the synthetic route of which is:
[0082] ;
[0083] Wherein, the transformation reagent is an alkaline aqueous solution, an alkaline alcohol solution or an alkaline alcohol-water mixture;
[0084] The R is -H, -CH 3 、-C 2 h 5 One of.
[0085] The conversion reagent is an aqueous solution of triethylamine, an alcohol solution of triethylamine or a mixed solution of alcohol and water of triethylamine.
[0086] 1) When the conversion reagent is an aqueous solution of triethylamine, its preparation method is:
[0087] Dissolve 4-trifluoromethylnicotinoyl chloride in tetrahydrofuran solution, add triethylamine and aminoacetonitrile hydrochloride; react at a temperature of 30-50 °C for 10 h, then add water to quench the reaction, extract with eth...
Embodiment 3-7
[0111] Example 3-7 The method of converting N-cyanomethylbis(trifluoromethyl)nicotinamide into flonicamid
[0112] Examples 3-7 are respectively a method for converting N-cyanomethylbis(trifluoromethyl)nicotinamide into flonicamid, which is similar to the preparation method described in Example 1, except that The technical parameters involved are different, as shown in the following table:
[0113]
[0114]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com