Application and medicines of benzamide compound
A technology of benzamides and compounds, applied in the direction of drug combinations, medical preparations containing active ingredients, anti-tumor drugs, etc., to achieve obvious inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
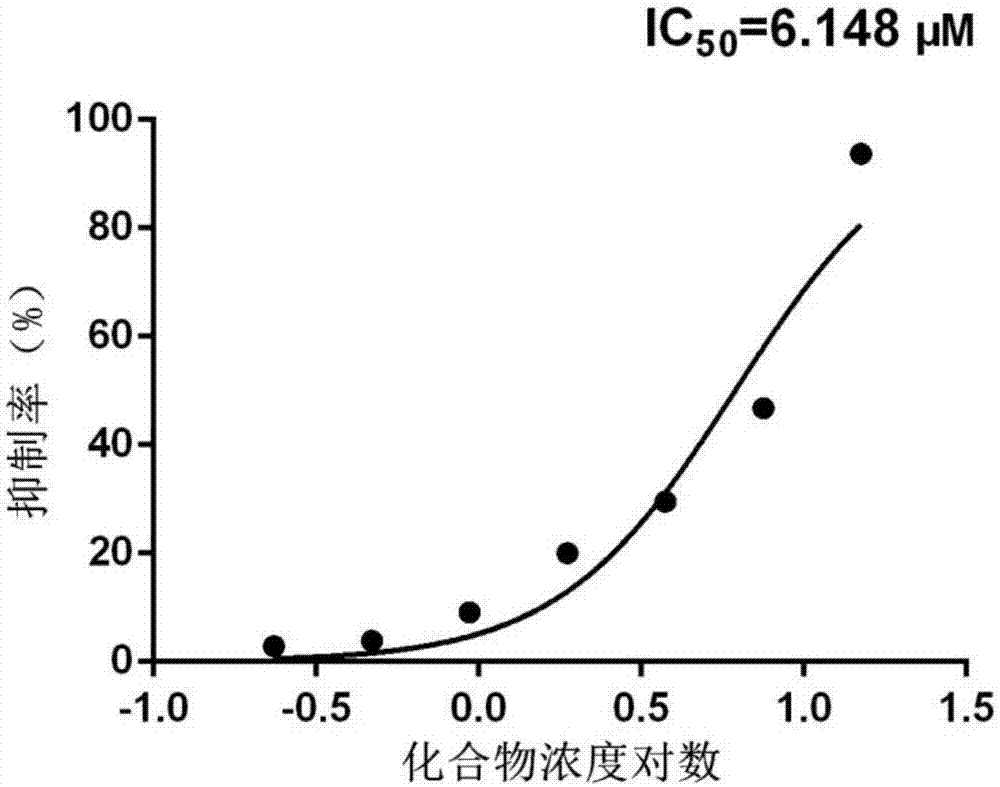
[0020] Example 1: The enzyme inhibitory activity test of compound 4-(3,5-dimethoxybenzoyl)-N-(4-hydroxy-5-isopropyl-2-methylphenyl)benzamide on AKR1B10 .
[0021] Experimental principle: Design based on the characteristic that the coenzyme NADPH is transformed into NADP+ with the reduction reaction of aldehyde and ketone substrates catalyzed by AKR1B10. Since NADPH has a characteristic maximum ultraviolet absorption peak at 340nm, and the product NADP+ absorbs very weakly at this wavelength, the degree of reduction of the coenzyme NADPH can be detected by detecting the decrease in the ultraviolet absorption value at 340nm, so as to obtain the speed of the enzyme-catalyzed reaction. In the initial stage of the reaction, the change curve of ultraviolet absorption at 340nm should be a straight line (zero-order reaction) theoretically, and the slope is proportional to the reaction speed.
[0022] When the compound was added, the reaction rate decreased, proving that the compound ...
Embodiment 2
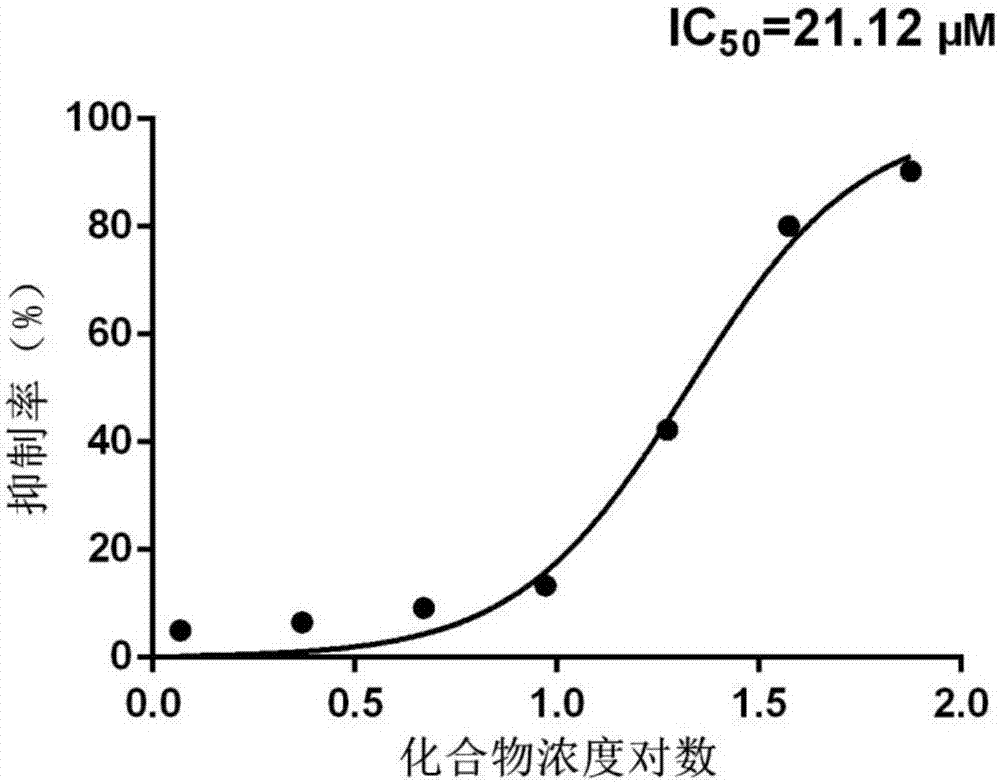
[0034]Example 2: The enzyme inhibitory activity test of compound 4-(3,5-dimethoxybenzoyl)-N-(4-hydroxy-5-isopropyl-2-methylphenyl)benzamide on AKR1B1 .
[0035] Experimental principle: Design based on the characteristic that the coenzyme NADPH is transformed into NADP+ with the reduction reaction of aldehyde and ketone substrates catalyzed by AKR1B1. Since NADPH has a characteristic maximum ultraviolet absorption peak at 340nm, and the product NADP+ absorbs very weakly at this wavelength, the degree of reduction of the coenzyme NADPH can be detected by detecting the decrease in the ultraviolet absorption value at 340nm, so as to obtain the speed of the enzyme-catalyzed reaction. In the initial stage of the reaction, the change curve of ultraviolet absorption at 340nm should be a straight line (zero-order reaction) theoretically, and the slope is proportional to the reaction speed.
[0036] When the compound was added, the reaction rate decreased, proving that the compound had...
Embodiment 3
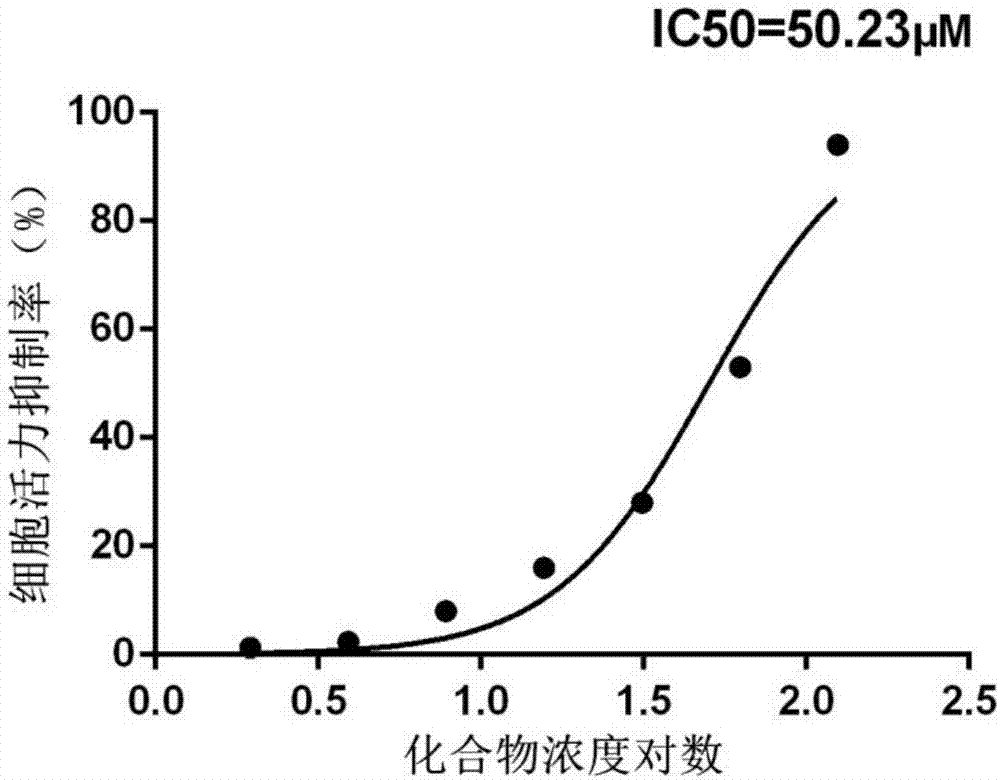
[0047] Example 3: MTT method detects the effect of compound 4-(3,5-dimethoxybenzoyl)-N-(4-hydroxy-5-isopropyl-2-methylphenyl)benzamide on liver cancer tumors in vitro Effects on Cellular HCC Survival.
[0048] Experimental principle: MTT, trade name thiazole blue, full name
[0049] 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. MTT colorimetry is a method for detecting cell viability. The detection principle is that succinate dehydrogenase in the mitochondria of living cells can reduce exogenous MTT to water-insoluble blue-purple crystalline formazan (Formazan) and deposit in the cells, while dead cells do not have this function. Dimethyl sulfoxide (DMSO) can dissolve formazan in cells, and its light absorption value is measured at a wavelength of 570nm with a microplate reader, which can indirectly reflect the number of surviving cells. Within a certain range, the amount of MTT crystal formation is directly proportional to the number of cell survival.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com