Pharmaceutical composition of ginsenosides RG5 and RZ1 and application of pharmaceutical composition in brain protection
A technology of ginsenosides and compositions, applied in the field of pharmaceutical compositions, which can solve the problems of low drug concentration, hindering chemical components, and affecting drug efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
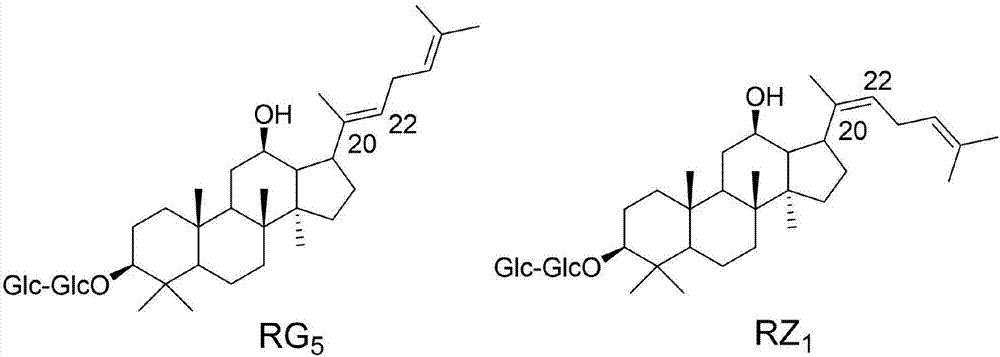
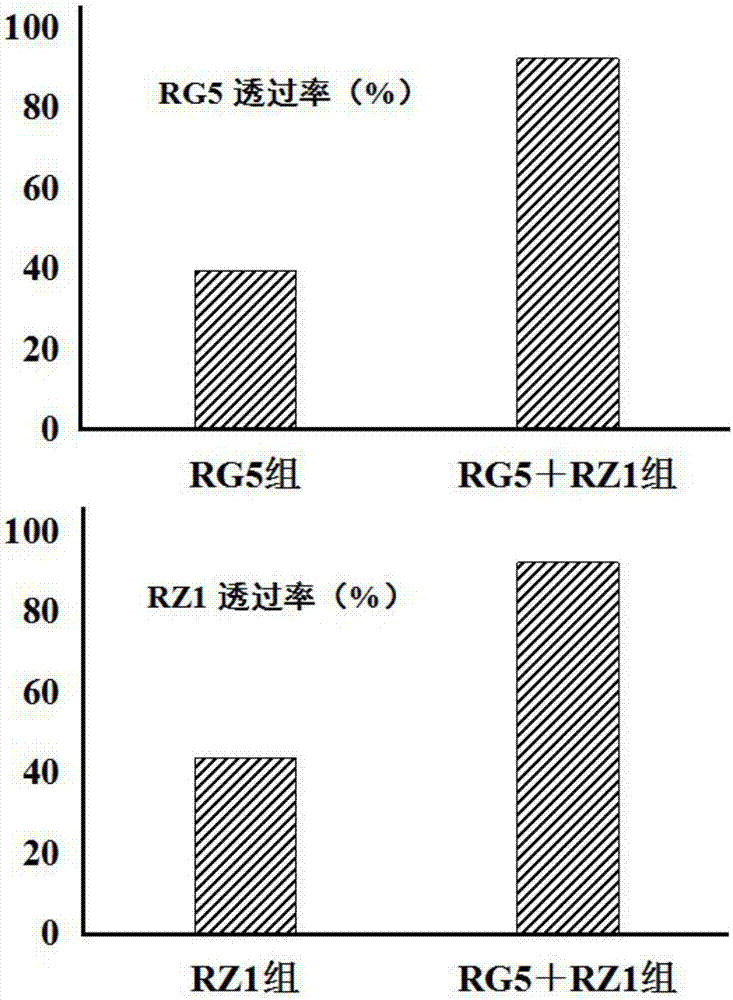
[0021] Example 1: Ginsenosides RG5 and RZ1 synergistically promote blood-brain barrier penetration
[0022] 1. Experimental method
[0023] 1. Culture and cryopreservation of mouse bEnd.3 cells
[0024] Mouse bEnd.3 cells (provided by Shanghai Cell Bank, Chinese Academy of Sciences) were cultured in DMEM high-glucose medium (containing 15% FBS, 1% 100 U / mL penicillin-streptomycin) at 37°C, 95% humidity, 5% CO 2 Culture in a constant temperature incubator, change the medium every other day, digest the cells with 0.25% trypsin when the cells grow to about 85% confluence in the culture flask, and passage them at a ratio of 1:2. Pre-prepared freezing solution (mixed solution of 10% DMSO and 90% FBS), stored in a 40°C refrigerator for pre-cooling. Take the cells in the logarithmic growth phase, discard the medium in the culture flask, wash 3 times with pre-warmed PBS, digest the cells with 0.25% trypsin, observe under the microscope, and add fresh pre-warmed medium when the cel...
Embodiment 2
[0045] Embodiment 2: Brain protection ginsenoside injection
[0046]The active ingredients are ginsenosides RG5 and RZ1, prepared with sterile water and surfactants.
Embodiment 3
[0047] Embodiment 3: brain protection ginsenoside tablet
[0048] The active ingredients are ginsenosides RG5 and RZ1, which are made into tablets with one or more solid, semi-solid or liquid excipients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com