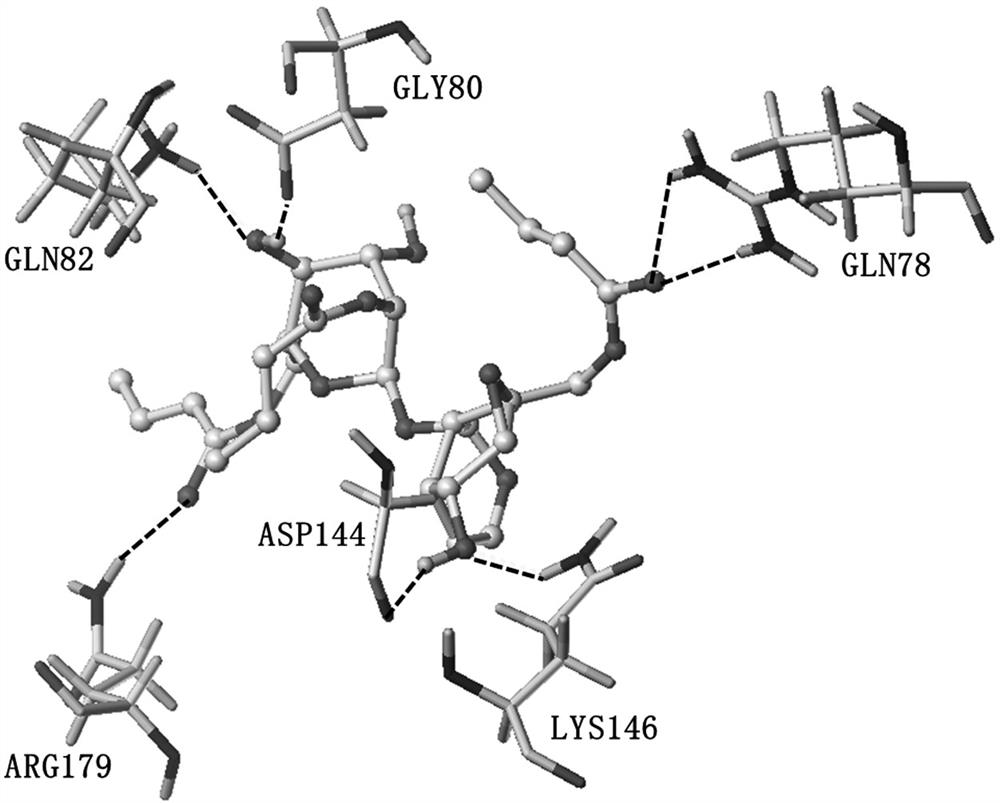
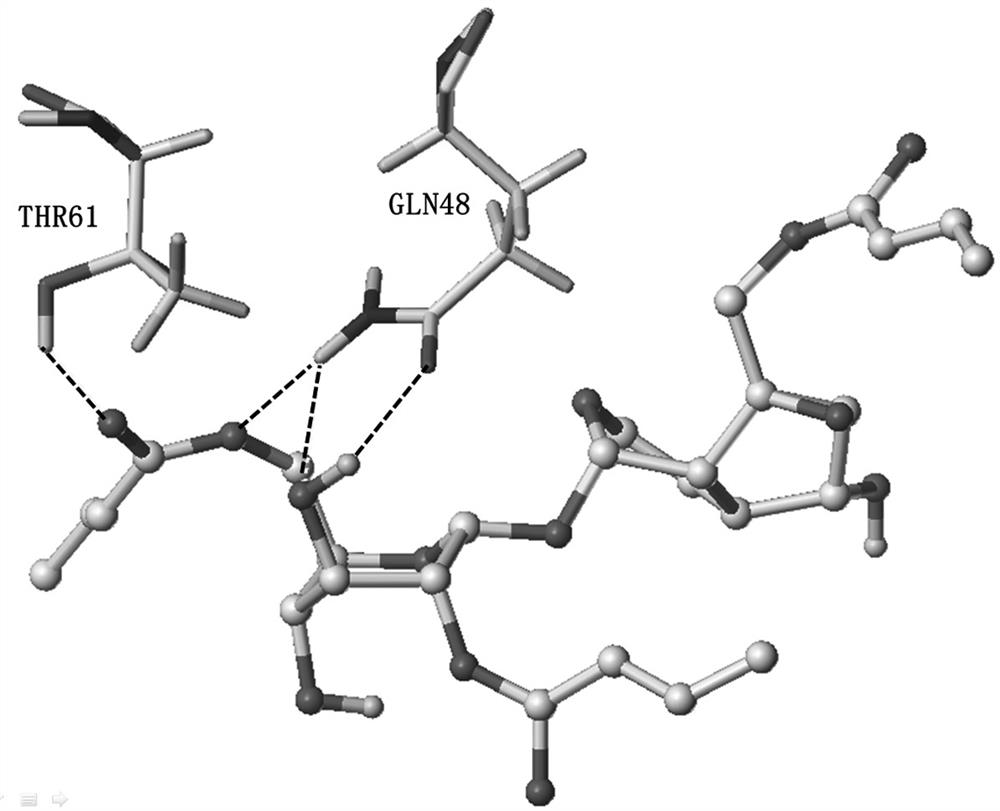
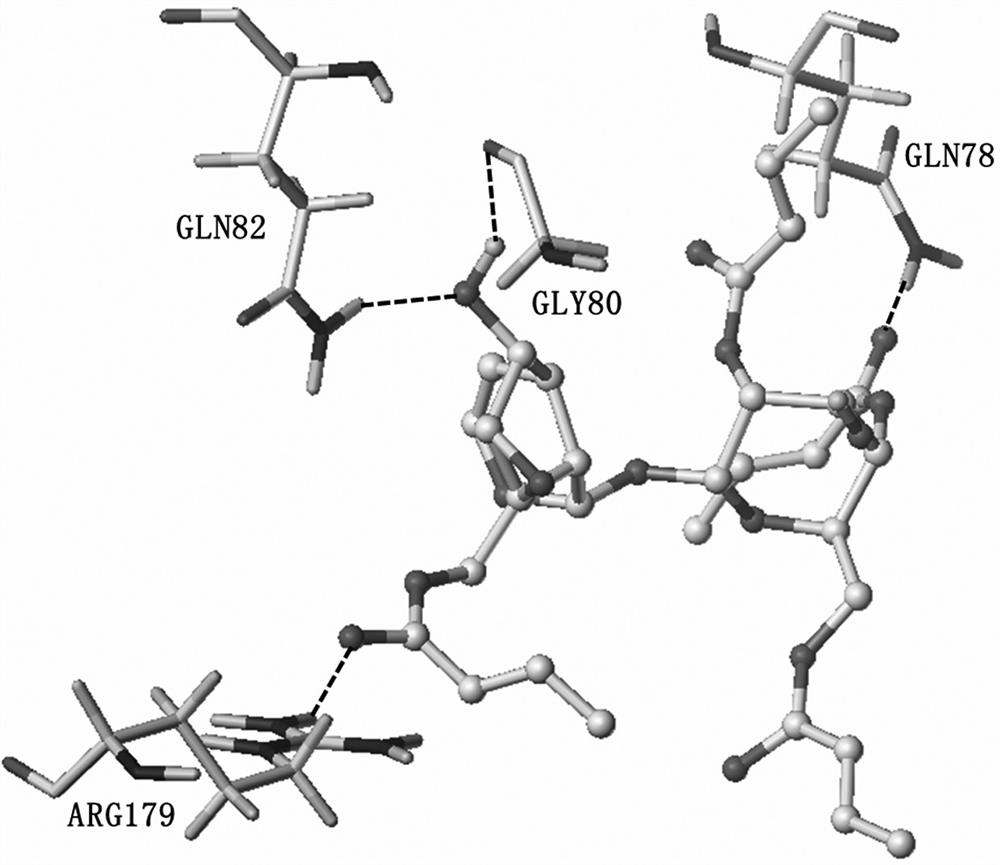
A kind of crotonylated catalpol derivative and its preparation method and application
A technology of crotonylation and derivatives, applied in the field of crotonylated catalpol derivatives and the preparation thereof, can solve the problems such as catalpol anhydride esterification derivatives that have not yet been found, achieve good anti-aging activity, reduce difficulty, low polar effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A crotonylated catalpol derivative obtained by esterifying catalpol with crotonic anhydride. During esterification, all hydroxyl groups of catalpol react with crotonic anhydride.
[0054] A method for preparing crotonylated catalpol derivatives, comprising the steps of:
[0055] 1) Dissolve 100mg (0.27mmol) catalpol in 10mL triethylamine, add 3.5mg catalyst 4-dimethylaminopyridine, under nitrogen protection, add 759μL (4.86mmol) crotonic anhydride, react at 60°C for 36h, The end of the reaction was monitored by the liquid phase, and the esterification yield was 99.16% detected by mass spectrometry, and the triethylamine was evaporated to dryness by rotary evaporation to obtain the product;
[0056] 2) Dissolve the product of step 1) in 40mL of dichloromethane, add 40mL of alkaline agent-saturated sodium bicarbonate solution, let stand for layering, discard the upper aqueous phase (add saturated sodium bicarbonate solution, let stand for layering, Discard the upper aqu...
Embodiment 2
[0059] A crotonylated catalpol derivative obtained by esterifying catalpol with crotonic anhydride. During esterification, some hydroxyl groups of catalpol react with crotonic anhydride.
[0060] A method for preparing crotonylated catalpol derivatives, comprising the steps of:
[0061] 1) Dissolve 0.27mmol of catalpol in 6.7mL of triethylamine, add 3.3mg of catalyst 4-dimethylaminopyridine, under nitrogen protection, add 1.63mmo of crotonic anhydride, react at 30°C for 24h, and monitor the end of the reaction by liquid phase , into the mass spectrometry detection, the esterification yield was 98.53%, and the triethylamine was evaporated to dryness by rotary evaporation to obtain the product;
[0062] 2) Dissolve the product of step 1) in 30mL of dichloromethane, add 20mL of alkaline agent-saturated sodium bicarbonate solution, let stand for layering, discard the upper aqueous phase (add saturated sodium bicarbonate solution, let stand for layering, Discard the upper aqueous...
Embodiment 3
[0065] A crotonylated catalpol derivative obtained by esterifying catalpol with crotonic anhydride. During esterification, some hydroxyl groups of catalpol react with crotonic anhydride.
[0066] A method for preparing crotonylated catalpol derivatives, comprising the steps of:
[0067] 1) Dissolve 100mg (0.27mmol) catalpol in 5mL triethylamine, add 3.1mg catalyst 4-dimethylaminopyridine, under nitrogen protection, add 3.24mmol crotonic anhydride, react at 90°C for 12h, liquid phase monitoring After the reaction was completed, the esterification yield was detected by mass spectrometry to be 97.21%, and the triethylamine was evaporated to dryness by rotary evaporation to obtain the product;
[0068] 2) Dissolve the product of step 1) in 40mL of dichloromethane, add 40mL of alkaline agent-saturated sodium bicarbonate solution, let stand for layering, discard the upper aqueous phase (add saturated sodium bicarbonate solution, let stand for layering, Discard the upper aqueous ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com