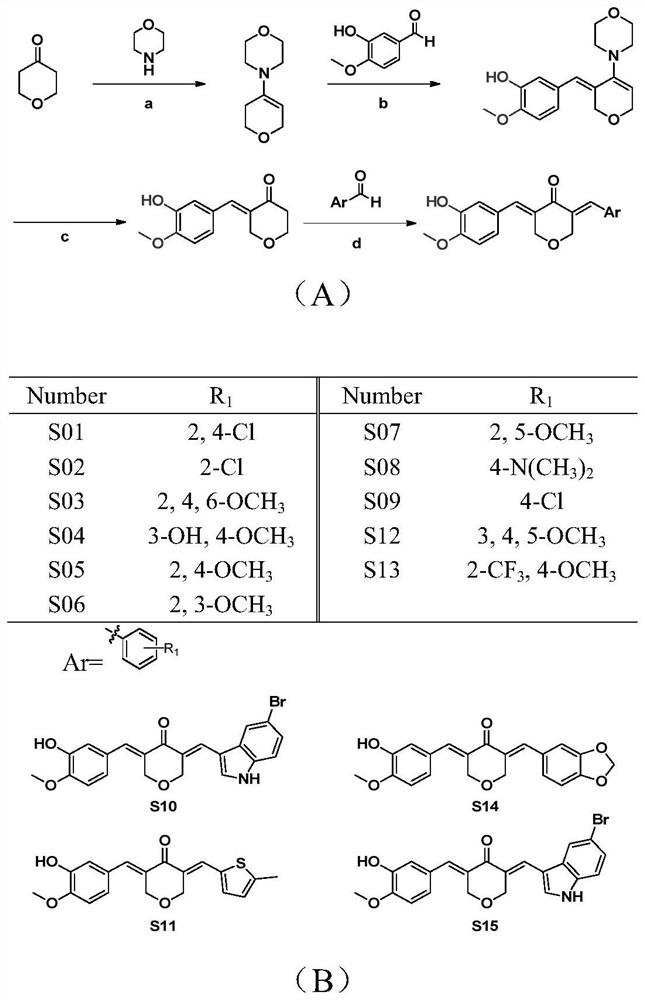
Asymmetric curcumin compounds and their application in the preparation of anti-gastric cancer drugs
A technology of curcumin and compounds, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound
[0029] Weigh 50mmoL of pyrone and 75mmoL of morpholine into a round bottom flask, add 50mL of reaction solvent cyclohexane and 1g of catalyst p-toluenesulfonic acid. After reflux at 90° C. for 4 h, the reaction solvent was removed by rotary evaporation to obtain a small amount of light yellow liquid. Add 25mmoL of 3-methoxy-4-hydroxybenzaldehyde to the above-mentioned round-bottomed flask containing light yellow liquid, add 30mL of absolute ethanol as a reaction solvent, and reflux at 78°C. Detected by TLC, the end point of the reaction was the appearance of a symmetrical by-product that was yellow at 365 nm. After the above reaction solution is cooled, add an appropriate amount of dilute hydrochloric acid to adjust the pH to 2-3, stir at room temperature to produce a precipitate, filter, and dry to obtain a crude intermediate product for later use.
[0030] Weigh each 1mmoL of the above-mentioned intermediate crude product and...
Embodiment 2
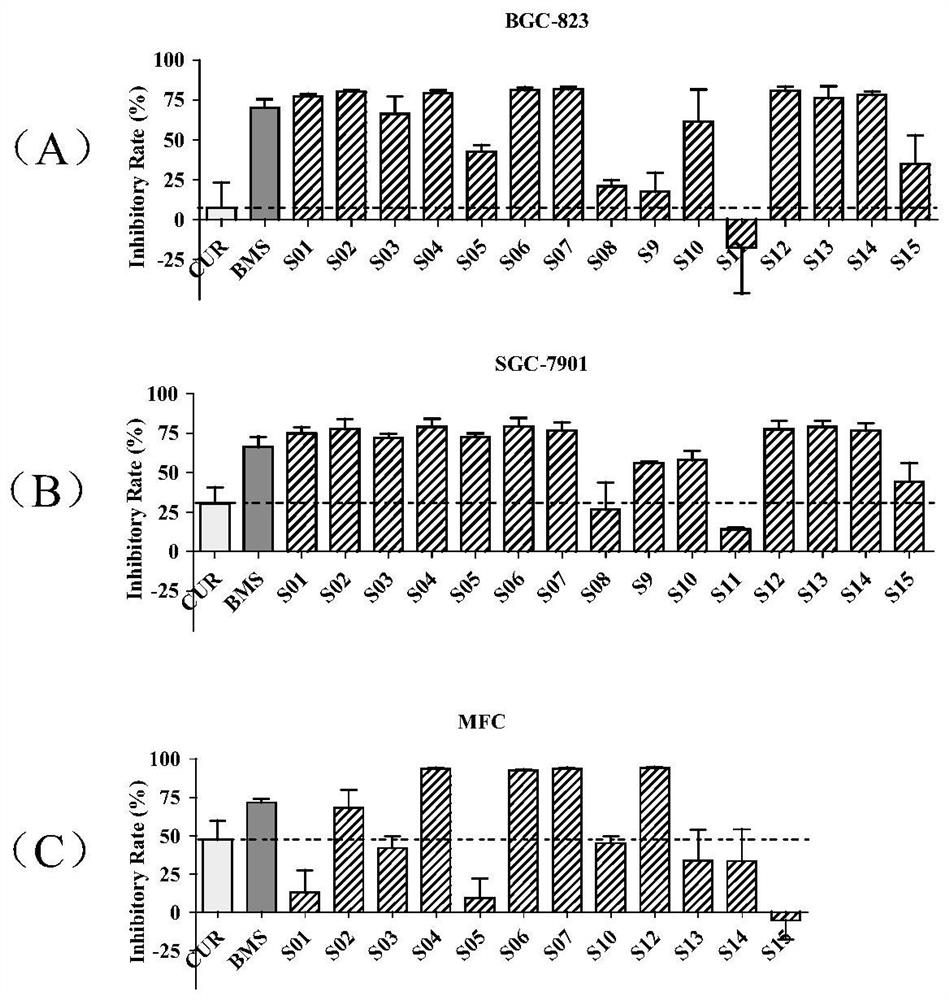
[0044] Example 2 Screening of S series compounds for growth inhibition of gastric cancer cells.
[0045] The inhibitory effect of curcumin analogues on the growth of gastric cancer cells was detected by MTT assay. The results of these analogs on gastric cancer cells BGC-823, SGC-7901 and MFC were as follows figure 2 A, B and C are shown. The inhibition rate of most of the compounds on the growth of three gastric cancer cell lines reached more than 60%. The inhibitory activity of compounds S01, S02, S04, S06, S07 and S12-14 on the proliferation of BGC-823 cells is higher than that of BMS-345541, and more than ten times that of curcumin. The inhibitory activity of compounds S01-07 and S12-14 on the proliferation of SGC-7901 cells was higher than that of BMS-345541, and more than twice that of curcumin. The inhibitory activity of compounds S04, S06, S07 and S12 on MFC cell proliferation was higher than that of BMS-345541 and more than twice that of curcumin. The four compoun...
Embodiment 3
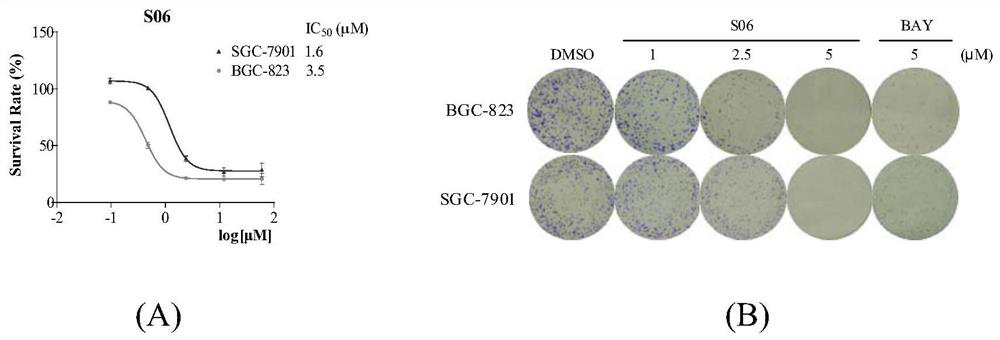
[0046] Example 3 Effect of compound S06 on the growth of gastric cancer cells.
[0047] The compound S06 with better activity was screened from the MTT experiment, and further biological evaluation was carried out. The IC of compound S06 on BGC-823 and SGC-7901 cell lines was measured 50 . The results showed that the IC of S06 on BGC-823 cells 50 IC of 3.51μM on SGC-7901 cells 50 1.55μM ( image 3 A). The effect of compounds on cell proliferation was studied by colony formation assay. Gastric cancer cells were treated with different concentrations of S06 (1, 2.5, 5 μM). The results showed that S06 could dose-dependently inhibit the formation of cell colonies ( image 3 B). There was almost no visible colony formation in the group treated with S06 at a concentration of 5 μM. The curative effect of S06 at 5μM was significantly better than that of the positive drug BAY11-7082 at the same concentration. It can be seen that compound S06 has medicinal prospects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com