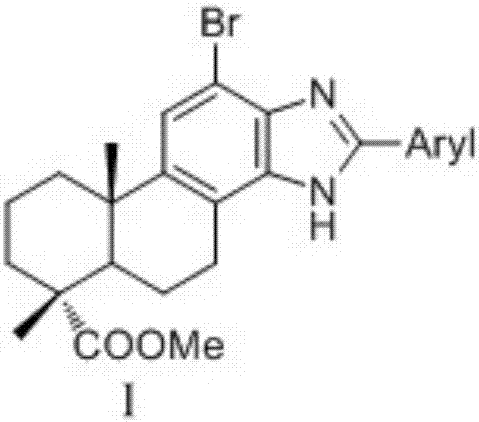
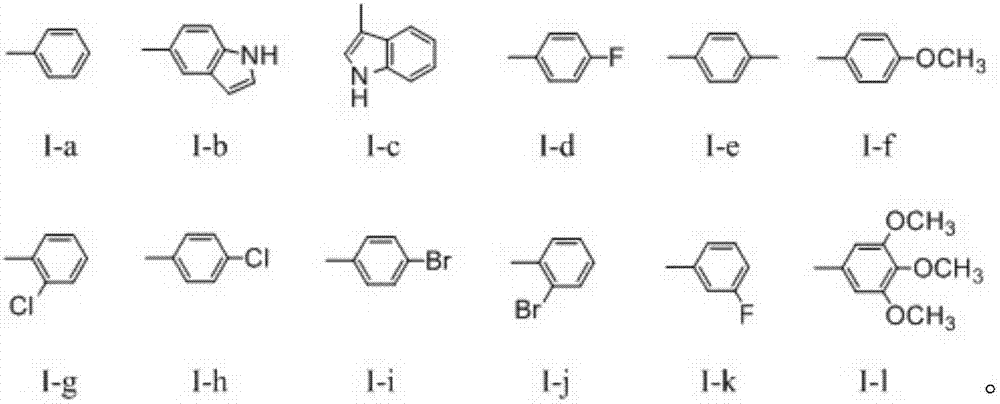
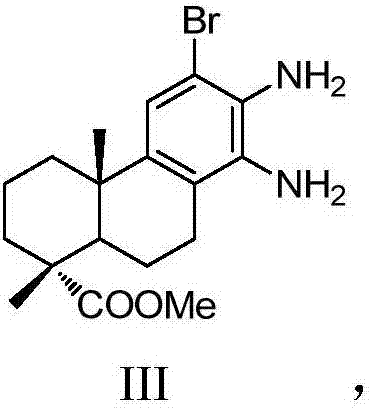
Dehydroabietic acid benzimidazole derivatives with antitumor activity as well as preparation method and application of dehydroabietic acid benzimidazole derivatives
A technology of dehydroabietic acid and benzimidazoles, which is applied in the fields of antineoplastic drugs, organic chemistry, and drug combinations, and can solve problems such as killing cells, infection, and bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Synthesis of Dehydroabietic Acid Benzimidazole Derivatives I-a
[0076] (1) dehydroabietic acid undergoes methyl esterification, bromination and double nitration reactions to obtain 12-bromo-13,14-dinitrodeisopropyl dehydrogenation methyl ester II;
[0077] (2) 12-bromo-13,14-dinitro-deisopropyl dehydroabietic acid methyl ester II is reduced by Fe / HCl to obtain 12-bromo-13,14-diamino-deisopropyl dehydroabietic acid methyl Ester III;
[0078] (3) In a three-necked flask, dissolve 0.66g (1.4998mmol) of 12-bromo-13,14-diaminodeisopropyl dehydroabietic acid methyl ester III in 30mL of absolute ethanol, and then add Add 0.24g (2.21mmol) of benzaldehyde, and then add 25.8mg (0.1499mmol) of p-toluenesulfonic acid. At 85°C, the mixture is stirred and refluxed for 24h under the protection of nitrogen. After the reaction, ethanol is removed by distillation under reduced pressure. Solvent, then want to add distilled water in the distillation residue, after extracting with ethyl ...
Embodiment 2
[0082] The difference between embodiment 2 and embodiment 1 is:
[0083] Synthesis of Dehydroabietic Acid Benzimidazole Derivatives I-b
[0084] In step (3), in a three-necked flask, 0.66 g (1.4998 mmol) of 12-bromo-13,14-diaminodeisopropyl dehydroabietic acid methyl ester III was dissolved in 30 mL of absolute ethanol, Then, 0.32 g (2.21 mmol) of indole-5-carboxaldehyde was added to the mixed solution, and then 25.8 mg (0.1499 mmol) of p-toluenesulfonic acid was added. At 85°C, the mixed solution was stirred and refluxed for 24 hours under the protection of nitrogen, and the reaction After the end, remove the ethanol solvent by distillation under reduced pressure, then add distilled water to the distillation residue, extract 2-3 times with ethyl acetate, then wash the organic phase with distilled water and saturated sodium chloride solution successively, and finally dry it over anhydrous sodium sulfate. Distill under reduced pressure, concentrate the organic phase, separate ...
Embodiment 3
[0088] The difference between embodiment 3 and embodiment 1 is:
[0089] Synthesis of Dehydroabietic Acid Benzimidazole Derivatives I-c
[0090] In step (3), in a three-necked flask, 0.66 g (1.4998 mmol) of 12-bromo-13,14-diaminodeisopropyl dehydroabietic acid methyl ester III was dissolved in 30 mL of absolute ethanol, Then, 0.32 g (2.21 mmol) of phenindole aldehyde was added to the mixed solution, and 25.8 mg (0.1499 mmol) of p-toluenesulfonic acid was added. At 85°C, the mixed solution was stirred and refluxed for 24 hours under the protection of nitrogen. After the reaction was completed, , remove the ethanol solvent by distillation under reduced pressure, then add distilled water to the distillation residue, extract 2-3 times with ethyl acetate, wash the organic phase with distilled water and saturated sodium chloride solution successively, and finally dry it with anhydrous sodium sulfate, Distill, concentrate the organic phase, separate and purify by silica gel column c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com