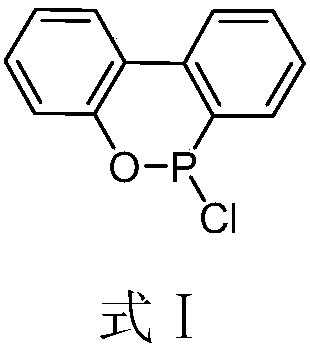
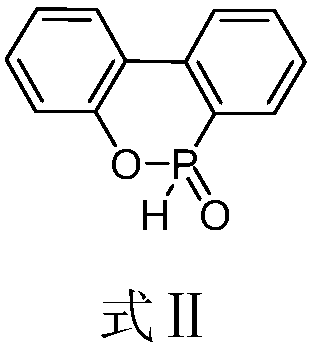
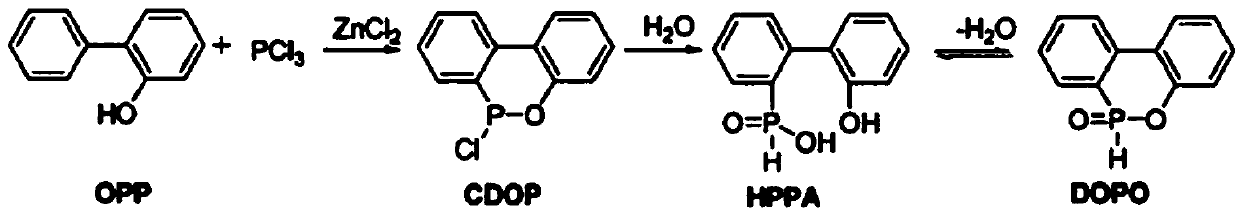
Preparation method of DOPO (9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) and intermediate of DOPO
An o-phenylphenol and compound technology, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, and organic chemistry, etc. problems, to achieve the effect of simplifying production operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation of DOPO by the method of the present invention
[0047] 1. Add 100g o-phenylphenol (1eq, 0.588mol), 1g anhydrous zinc chloride (0.0125eq, 0.0073mol) and 80.7g phosphorus trichloride (1eq, 0.588mol) into a 500mL four-necked flask, and heat up to After reacting at 100° C. for 2 hours, continue to raise the temperature to 160° C. for 2 hours. The reaction was complete as detected by HPLC, and the reaction solution was directly used for the next reaction.
[0048] 2. Add 150 g of toluene and 5 g of diatomaceous earth to the reaction liquid in the previous step, stir at room temperature for 0.5 h, and filter to obtain a colorless clear liquid. 50 g of purified water was added to the filtrate, the temperature was raised to reflux for 4 h, and the reaction was detected by TLC to be complete. Cool down to 0°C, stir vigorously to precipitate a white solid, filter, wash the filter cake twice with 50 g of purified water, the filter cake is detected as a HPPA...
Embodiment 2
[0051] Example 2 Preparation of DOPO by the method of the present invention
[0052] 1. Add 100g of o-phenylphenol (1eq, 0.588mol), 1g of anhydrous zinc chloride (0.0125eq, 0.0073mol) and 112.98g of phosphorus trichloride (1.4eq) into a 500mL four-necked flask, and heat up to 80°C After reacting for 2 hours, continue to heat up to 180° C. for 2 hours, and the reaction is complete as detected by HPLC, and the reaction solution is directly used for the next reaction.
[0053]2. Add 150 g of toluene and 5 g of diatomaceous earth to the reaction liquid in the previous step, stir at room temperature for 0.5 h, and filter to obtain a colorless clear liquid. 50 g of purified water was added to the filtrate, the temperature was raised to reflux for 4 h, and the reaction was detected by TLC to be complete. Cool down to 0°C, stir vigorously to precipitate a white solid, filter, wash the filter cake twice with 50 g of purified water, collect the filter cake, and directly use it for the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com