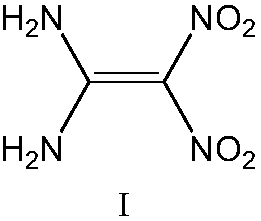
Method for synthesizing 1,1-diamino-2,2-dinitroethylene
A technology of dinitroethylene and synthesis methods, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, etc., which can solve the problems of severe heat release in nitration reactions, large environmental pollution, and high processing costs, and achieve processing costs. High, environmental pollution, good heat dissipation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of 1,1-diamino-2,2-dinitroethylene with nitric-sulfur mixed acid directly prepared
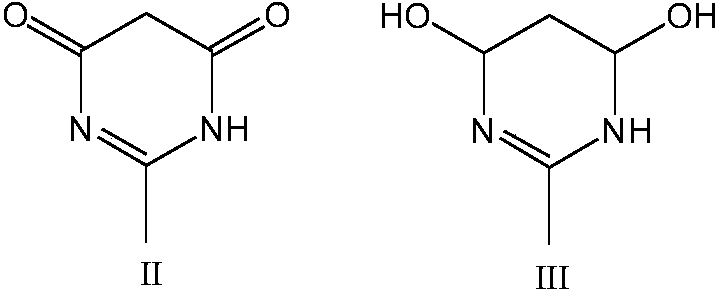
[0019] At 15°C, 50.0g of sulfuric acid with a mass fraction of 98% was added to the reaction flask, and then 100.0g of nitric acid with a mass fraction of 98% was added dropwise. After the dropwise addition, the temperature was lowered to 0°C, and 10.0g of 2 -Methyl-4,6-pyrimidinedione, then heated up to 15°C for 3 hours, cooled to 0°C after the reaction and filtered, collected the nitrating acid filtrate, added the filter cake to 450g of ice water, filtered and dried to obtain 1, 10.2 g of 1-diamino-2,2-dinitroethylene, yield 86.8%.
[0020] Structure Identification:
[0021] IR spectrum (KBr pellet / cm -1 ): υ NH :3422, 3406, 3330, 3298; δ NH :1636; υ C=C :1608; υ as-NO2 :1517; υ s-NO2 :1226;
[0022] Elemental analysis (C 2 h 4 N 4 o 4 ): Theoretical: C 16.22, H 2.72, N 37.84 Found: C 16.56, H 2.429, N 37.71
[0023] 1 H NMR (DMSO-d 6 , δ, ppm): 8.781;
[0...
Embodiment 2
[0027] Preparation of 1,1-diamino-2,2-dinitroethylene from nitric-sulfur mixed acid prepared from recycled nitrating acid filtrate
[0028] At 20°C, weigh 62.5g of the nitrating acid filtrate that circulates once, add successively 25.0g of oleum with a mass fraction of 50% and 62.5g of nitric acid with a mass fraction of 98%, to prepare 150.0g of nitric acid mixed acid; Cool the sulfur mixed acid to 0°C, add 10.0g of 2-methyl-4,6-pyrimidinedione in batches under stirring, then raise the temperature to 15°C for 3 hours, cool down to 0°C after the reaction and filter, collect the nitrating acid filtrate, filter The cake was added to 450 g of ice water, filtered and dried to obtain 10.2 g of 1,1-diamino-2,2-dinitroethylene with a yield of 86.8%.
[0029] Structure Identification:
[0030] IR spectrum (KBr pellet / cm -1 ): υ NH :3424, 3408, 3331, 3298; δ NH :1638; υC=C :1608; υ as-NO2 :1518; υ s-NO2 :1225;
[0031] Elemental analysis (C 2 h 4 N 4 o 4 ): Theoretical: C 16...
Embodiment 3
[0036] Preparation of 1,1-diamino-2,2-dinitroethylene from nitric-sulfur mixed acid prepared from nitrating acid filtrate which has been circulated three times
[0037] At 0°C, weigh 62.5 g of the nitrating acid filtrate circulated three times, add successively 25.0 g of oleum with a mass fraction of 50% and 62.5 g of nitric acid with a mass fraction of 98%, to prepare 150.0 g of nitric acid mixed acid; Cool the sulfuric acid to -10°C, add 10.0g of 2-methyl-4,6-pyrimidinedione in batches under stirring, then raise the temperature to 10°C for 3 hours, cool down to 0°C after the reaction and filter, collect the nitrating acid filtrate, The filter cake was added to 450 g of ice water, filtered and dried to obtain 9.8 g of 1,1-diamino-2,2-dinitroethylene with a yield of 83.4%.
[0038] Analytical data confirmed that the compound obtained was 1,1-diamino-2,2-dinitroethene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com