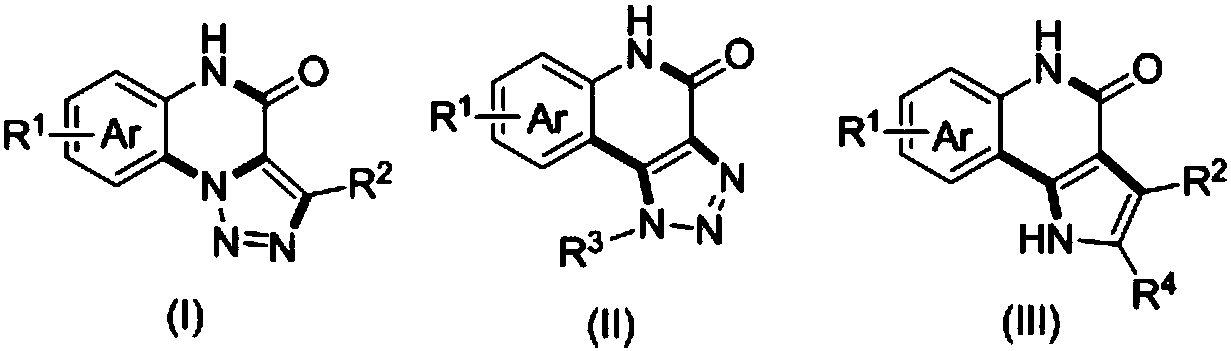
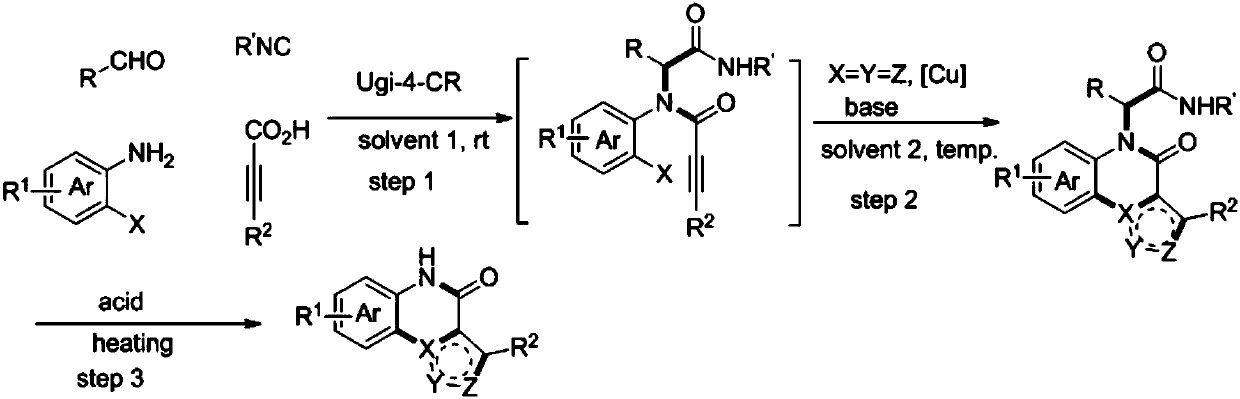
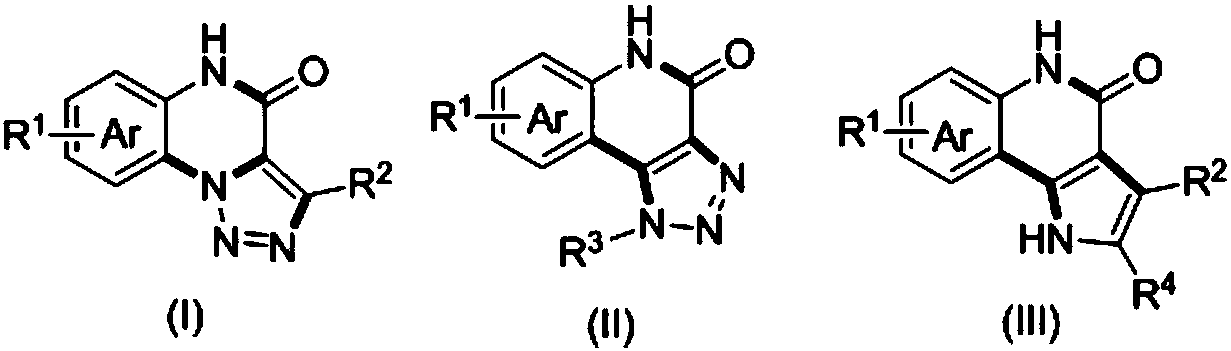
Method for compounding nitrogenous polynary heterocyclic compound
A technology for heterocyclic compounds and compounds, which is applied in the field of synthesizing nitrogen-containing polyheterocyclic compounds, can solve problems such as no method has been found, and achieve the effects of good functional group compatibility, simple and efficient preparation method, and simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the synthesis of compound 6a
[0038]
[0039] Compounds 1a (1.0mmol) and 2a (1.0mmol) were stirred in EtOH for 0.5h, then 3a (1.0mmol) was added to continue stirring at room temperature for 0.5h, then 4a (1.0mmol) was added, and the four components were stirred at room temperature for 24h. Add NaN after spin off the solvent 3 (1.2mmol) and CuI (5mol%), DMSO was added under the protection of argon, and the reaction was complete at 90°C (18h). Water was added and extracted with ethyl acetate, the organic phases were combined, washed with water, dried, and concentrated to obtain the crude compound 5a.
[0040] Dissolve compound 5a in trifluoroacetic acid at 80°C and stir until the reaction is complete (28h), add saturated NaHCO 3 The pH value was adjusted to 8-9, and ethyl acetate was added to separate and extract the aqueous phase, and then the organic phases were combined, washed with water, dried, concentrated and filtered to obtain a yellow solid, n...
Embodiment 2
[0041] Embodiment 2: the synthesis of compound 6b
[0042]
[0043] Dissolve compound 1b (1.0mmol) and 2b (1.0mmol) in MeOH and stir for 0.5h, then add 3a (1.0mmol) and continue stirring for 0.5h, then add 4b (1.0mmol), and stir for 24h. Spin off the solvent, add NaN 3 (1.2mmol) and CuBr (10mol%), DMF was added under argon protection, and the reaction was complete at 100°C (4h). Water was added and extracted with ethyl acetate. The organic phases were combined, washed with water, dried, and concentrated to obtain the crude compound 5b.
[0044] Dissolve compound 5b in toluene, add hydrochloric acid (2mmol) and stir at 90°C until the reaction is complete (6-8h), add NaOH to adjust the pH value to 8-9, add ethyl acetate to separate and extract the aqueous phase, combine the organic phases, wash with water and Dry, concentrate and filter to obtain a yellow solid, namely compound 6b, with a yield of 50%. The characterization data of compound 6b are: 1 H NMR (400MHz, DMSO) ...
Embodiment 3
[0045] Embodiment 3: the synthesis of compound 6c
[0046]
[0047] Compounds 1c (1mmol) and 2c (1mmol) were dissolved in i-PrOH and stirred for 0.5h, then 3b (1mmol) was added and stirred for 0.5h, then 4a (1mmol) was added, and the four components were stirred for 24h. Spin off the solvent, add NaN 3 (1.2mmol) and CuCl (20mol%), DMSO was added under the protection of argon, and the reaction was complete at 100°C (6h). Water was added, and the aqueous phase was extracted with ethyl acetate, and the organic phases were combined, washed with water, dried, and concentrated to obtain a crude compound 5c.
[0048] Dissolve compound 5c in DMSO, add sulfuric acid (2mmol) and stir at 80°C until the reaction is complete (6-8h), add NaOH to adjust the pH value to 8-9, add ethyl acetate to extract the aqueous phase, combine the organic phase, and wash the organic phase with water. phase and dried, concentrated and filtered to obtain a yellow solid, namely compound 6c, with a yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com