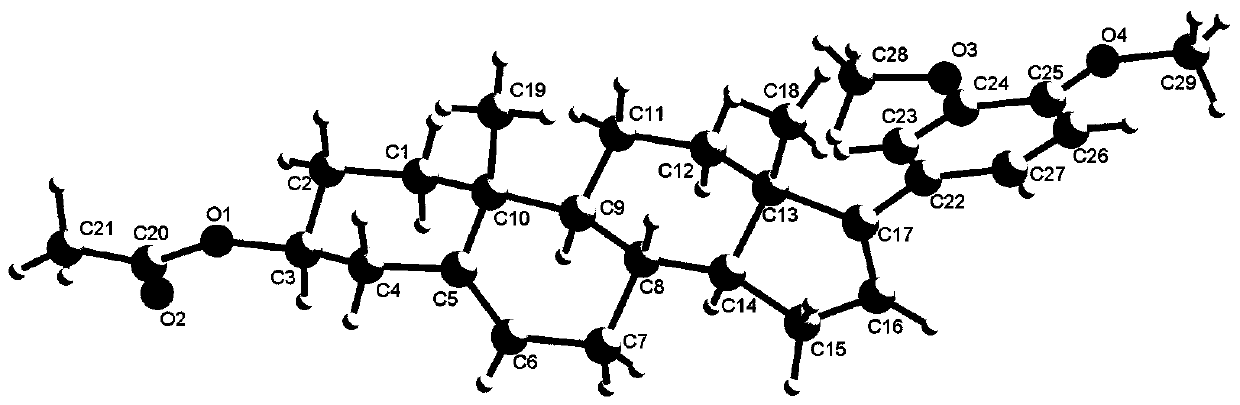
Synthesis method of 3 beta-acetoxy-17-aryl androsta-5,16-diene
A technology of acetoxyandrostane and acetoxyl, applied in the field of synthesis of 3β-acetoxy-17-arylandrost-5,16-diene, can solve the problems of unfavorable pharmaceutical production industrial implementation, expensive metal catalysts, initial Rare raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
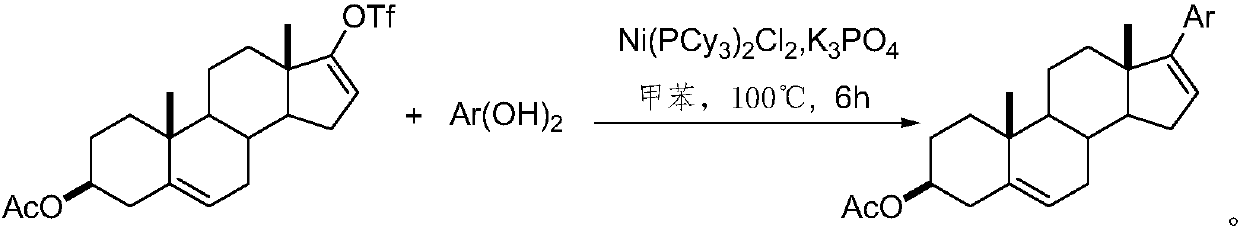
[0016] A method for synthesizing 3β-acetyloxy-17-arylandrosta-5,16-diene of the present invention comprises the following steps: using 17-trifluoromethanesulfonyloxy-3β-acetyloxyandrost-5, 16-diene as raw material, toluene as solvent, in nickel catalyst Ni(PCy 3 ) 2 Cl 2 Under the influence of reaction, 3β-acetoxy-17-aryl androst-5,16-diene compound was obtained after reaction for 6h:
[0017]
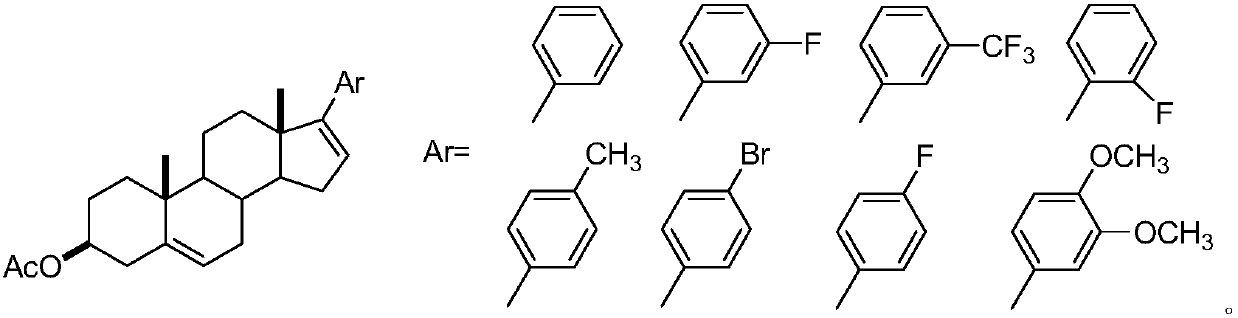
[0018] The 3β-acetoxy-17-arylandrost-5,16-diene compound of the present invention includes the following structural formula:
[0019]
[0020] Reagent and condition of the present invention: compound (0.924g, 2.0mmol), Ni(PCy 3 ) 2 Cl 2 (0.07g, 0.1mmol), K 3 PO 4 (1.30g, 6.0mmol), arylboronic acid (3.0mmol), toluene (15mL), 100°C, 6h; yield: 52-85%.
[0021] test instrument
[0022] Infrared spectrum analysis: Bruker tensor 27 infrared spectrometer, KBr tablet method, detection range is 4000~400cm -1 ; Melting point measurement: WRR melting point instrument (Shanghai Pr...
Embodiment 1
[0023] Example 1: Synthesis of 3β-acetoxy-17-phenylandrost-5,16-diene (3a)
[0024]
[0025] Weigh 0.924g (2.0mmol) of 17-trifluoromethanesulfonyloxy-3β-acetoxy-androst-5,16-diene (1), catalyst Ni (PCy 3 ) 2 Cl 2 0.07g (0.1mmol), K 3 PO 4 1.30g (6.0mmol) and 0.366g (3.0mmol) of phenylboronic acid were added to 15mL of toluene, and stirred at room temperature for 5min. Then, under the protection of nitrogen, the reaction was stirred at 100° C. for 6 h, and the reaction progress was tracked by TLC (EtOAc / hexanes 1:8). After the reaction was completed, the toluene solvent was evaporated under vacuum, and the residue was passed through CH 2 Cl 2 Extract 2 times, 10 mL each time, combine the organic phases, wash the organic phases with water and saturated brine twice, 5 mL each time, anhydrous Na 2 SO 4 dry. Evaporate CH 2 Cl 2 Solvent, the crude product was purified by flash chromatography (silica gel, EtOAc / hexanes, 1 / 40) to obtain a white solid product, yield: 85%...
Embodiment 2
[0026] Example 2: Synthesis of 3β-acetoxy-17-(3-fluorophenyl)androst-5,16-diene (3b)
[0027]
[0028] Weigh 0.924g (2.0mmol) of 17-trifluoromethanesulfonyloxy-3β-acetoxy-androst-5,16-diene (1), catalyst Ni (PCy 3 ) 2 Cl 2 0.07g (0.1mmol), K 3 PO 4 1.30 g (6.0 mmol) and 0.42 g (3.0 mmol) of 3-fluorophenylboronic acid were added to 15 mL of toluene, and stirred at room temperature for 5 min. Then, under the protection of nitrogen, the reaction was stirred at 100° C. for 6 h, and the reaction progress was tracked by TLC (EtOAc / hexanes 1:8). After the reaction was completed, the toluene solvent was evaporated under vacuum, and the residue was passed through CH 2 Cl 2 Extract 2 times, 10 mL each time, combine the organic phases, wash the organic phases with water and saturated brine twice, 5 mL each time, anhydrous Na 2 SO 4 dry. Evaporate CH 2 Cl 2 Solvent, the crude product was purified by flash chromatography (silica gel, EtOAc / hexanes, 1 / 40) to obtain a light ye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com