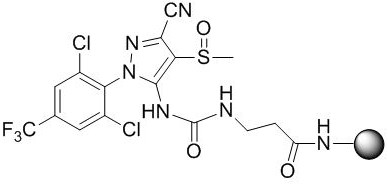
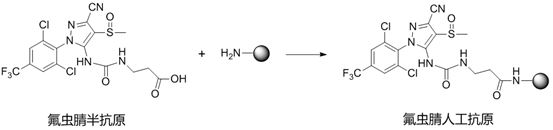
A kind of fipronil hapten and its synthesis method, artificial antigen, antibody and application
A synthesis method, artificial antigen technology, applied in chemical instruments and methods, animal/human proteins, instruments, etc., can solve the problems of sensitivity, specificity and precision need to be improved, and achieve high sensitivity and precision, high synthesis purity , good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A synthetic method of fipronil hapten, comprising the following steps:
[0042] S1. Reaction of fipronil structural analogs with phenyl chloroformate to synthesize intermediate I, specifically:
[0043] Weigh 4.37g of fipronil structural analogues and dissolve in acetone, cool down in an ice bath, slowly add 1.88g of phenyl chloroformate dropwise, and then rise to 25°C to react overnight. The reaction solution was concentrated to give [5-cyano-2-(2,6-dichloro-4-trifluoromethyl-phenyl)-4-methylsulfoxide-2H-pyrazol-3-yl]- Phenyl carbamate (Intermediate I). The intermediate I can be directly carried out in the next step without purification.
[0044] S2. reacting the intermediate I with tert-butyl allanine to synthesize the intermediate II, specifically:
[0045] Dissolve 0.50g of intermediate I in 30mL of tetrahydrofuran, add 0.01g of DBU (1,8-diazabicycloundec-7-ene), 1.21mL of triethylamine, 0.73g of tert-alanine Butyl ester, the reaction solution was heated to 65°C...
Embodiment 2
[0051] Specifically with embodiment 1, the difference is:
[0052] When synthesizing the fipronil hapten, in step S1, weigh 4.40 g of the fipronil structural analogue and dissolve it in acetone, cool down in an ice bath, slowly add 1.73 g of phenyl chloroformate dropwise, and then rise to 40° C. to react overnight.
[0053] When synthesizing the fipronil hapten, in step S2, dissolve 0.50g of intermediate I in 30mL of tetrahydrofuran, add 0.01g of DBU, 1.21mL of triethylamine, and 0.73g of tert-butyl 3-aminopropionate in sequence, and heat up the reaction solution Reaction at 50°C for 12h.
[0054] When synthesizing the fipronil hapten, in step S3, weigh 0.55 g of intermediate II and dissolve it in 10 mL of dichloromethane, add 10 mL of trifluoroacetic acid and stir at room temperature for 2 h. The purity of the synthesized fipronil hapten was 99.6%.
[0055] When synthesizing fipronil artificial antigen, weigh 10 mg of fipronil hapten and dissolve it in 200 μL of DMF, add 7....
Embodiment 3
[0057] Specifically with embodiment 1, the difference is:
[0058] When synthesizing the fipronil hapten, in step S1, weigh 4.50 g of the fipronil structural analogue and dissolve it in acetone, cool down in an ice bath, slowly add 1.61 g of phenyl chloroformate dropwise, and react overnight at 0°C.
[0059] When synthesizing the fipronil hapten, in step S2, dissolve 0.50g of intermediate I in 30mL of tetrahydrofuran, add 0.01g of DBU, 1.21mL of triethylamine, and 0.73g of tert-butyl 3-aminopropionate in sequence, and heat up the reaction solution Reaction at 80°C for 12h.
[0060] When synthesizing the fipronil hapten, in step S3, weigh 0.55 g of intermediate II and dissolve it in 15 mL of dichloromethane, add 15 mL of trifluoroacetic acid and stir at room temperature for 2 h. The purity of the synthesized fipronil hapten was 99.7%.
[0061] When synthesizing the fipronil artificial antigen, weigh 10 mg of the fipronil hapten and dissolve it in 200 μL of DMF, add 7.7 mg of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com