N-substituted imidazole carboxylate compound, preparation method and use thereof
一种酯类化合物、咪唑羧酸的技术,应用在有机化学、药物组合、麻醉剂等方向,能够解决弱中枢抑制活性等问题,达到降低副作用、肌肉震颤的几率低、皮质功能恢复快的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
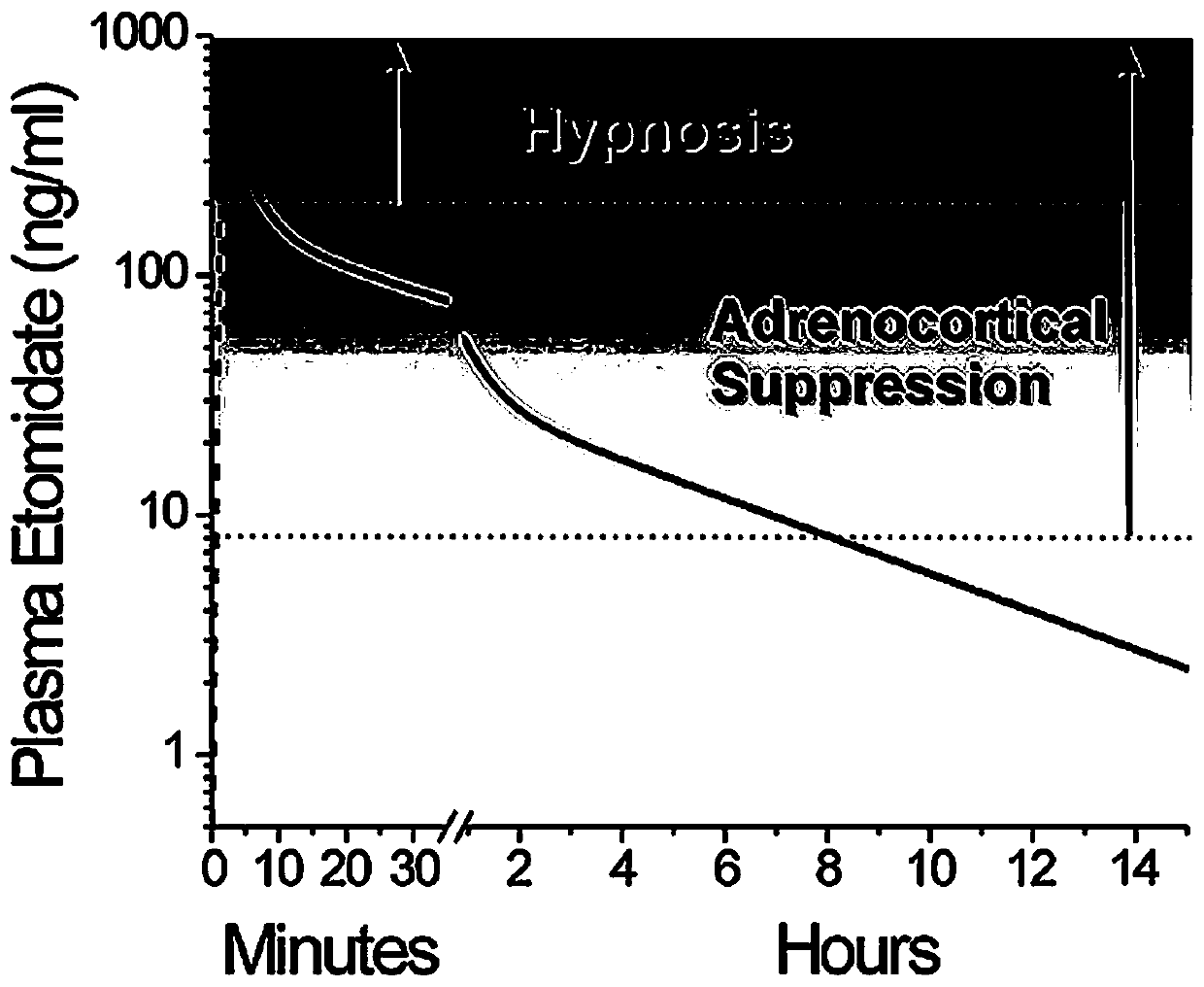
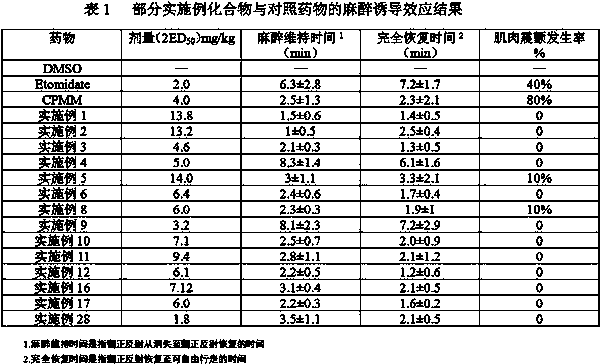
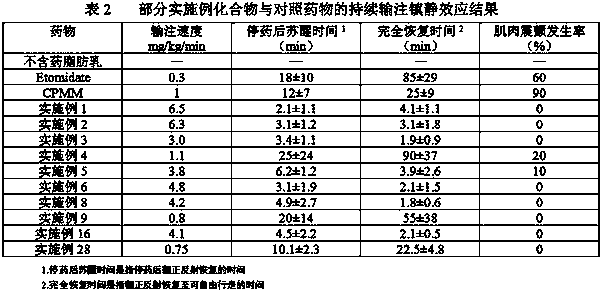
Image
Examples
Embodiment 1
[0035] Add 1.29 g of chloromethyl chloroformate (CAS: 22128-62-7) into 30 mL of anhydrous dichloromethane, add 0.36 g of anhydrous methanol, drop in 1.6 g of pyridine under cold water cooling, and stir for 2 hours. Add 50 mL of dichloromethane, and wash the organic layer twice with 2N hydrochloric acid, then once with water, separate the organic layer, and dry over anhydrous sodium sulfate overnight. After filtration, the filtrate was evaporated to dryness to obtain 1.15 g of crude methyl chloromethyl carbonate, which was directly used in the next reaction.
[0036] Dissolve 216 mg of etomidate acid (CAS: 56649-48-0) in 20 mL of DMF, add 136 mg of methyl chloromethyl carbonate, add 275 mg of potassium carbonate, and stir at room temperature for 3 hours. After the reaction solution was filtered, it was poured into 150 mL of water, extracted with 100 mL of dichloromethane, the organic layer was separated, dried overnight with anhydrous sodium sulfate, the filtrate was filtered t...
Embodiment 2
[0042] Add 1.29 g of chloromethyl chloroformate (CAS: 22128-62-7) into 30 mL of anhydrous dichloromethane, add 0.48 g of absolute ethanol, drop in 1.6 g of pyridine under cold water cooling, and stir for 2 hours. Add 50 mL of dichloromethane, and wash the organic layer twice with 2N hydrochloric acid, then once with water, separate the organic layer, and dry over anhydrous sodium sulfate overnight. After filtration, the filtrate was evaporated to dryness to obtain 1.19 g of crude ethyl chloromethyl carbonate, which was directly used in the next reaction.
[0043] Dissolve 216 mg of etomidate acid (CAS: 56649-48-0) in 20 mL of DMF, add 165 mg of ethyl chloromethyl carbonate, add 275 mg of potassium carbonate, and stir at room temperature for 3 hours. After the reaction solution was filtered, it was poured into 150 mL of water, extracted with 100 mL of dichloromethane, the organic layer was separated, dried overnight with anhydrous sodium sulfate, the filtrate was filtered the n...
Embodiment 3
[0049] Add 1.29 g of chloromethyl chloroformate (CAS: 22128-62-7) into 30 mL of anhydrous dichloromethane, add 0.66 g of isopropanol, drop in 1.6 g of pyridine under cold water cooling, and stir for 2 hours. Add 50 mL of dichloromethane, and wash the organic layer twice with 2N hydrochloric acid, then once with water, separate the organic layer, and dry over anhydrous sodium sulfate overnight. After filtration, the filtrate was evaporated to dryness to obtain 1.28 g of crude isopropyl chloromethyl carbonate, which was directly used in the next reaction.
[0050] Dissolve 216mg of etomidate acid in 20mL of DMF, add 155mg of isopropyl chloromethyl carbonate, add 275mg of potassium carbonate, stir at room temperature for 3 hours, and spot the plate. The basic reaction of raw materials is complete. After the reaction solution was filtered, it was poured into 150 mL of water, extracted with 100 mL of dichloromethane, the organic layer was separated, dried overnight with anhydrous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com