Complex probiotic for treating and preventing allergic diseases as well as preparation method and preparation of complex probiotic
A technology for compounding probiotics and allergic diseases, applied in allergic diseases, respiratory diseases, bacteria used in food preparation, etc., can solve the problems of immaturity development, etc. Effects of abundance and diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Present embodiment formula is as follows:
[0044] Strain Composition 0.05g
[0045] fructooligosaccharides 1.45g
[0046] Wherein the formula (weight composition) of bacterial strain composition is as follows:
[0047]
[0048] The preparation method of this compound probiotic is as follows:
[0049] The preparation method of above-mentioned composite probiotics is characterized in that comprising the following steps:
[0050] 1) Select a single colony of Lactobacillus salivarius, Lactobacillus salivarius, Bifidobacterium animalis, Lactobacillus johnsonii, Lactobacillus paracasei, and Lactobacillus reuteri to inoculate into a 2L culture bottle, and let stand at 35°C for 24 hours;
[0051] 2) Then move to the fermenter to carry out fermentation culture in the presence of culture medium, and scale up the culture to 200L within 24 hours at 35°C;
[0052] 3) Continue the scale-up culture in the presence of the culture medium in the fermenter, and scale up to 2T with...
Embodiment 2
[0058]Present embodiment formula is as follows:
[0059] Strain Composition 0.05g
[0060] fructooligosaccharides 1.45g
[0061] Wherein the formula (weight composition) of bacterial strain composition is as follows:
[0062]
[0063] All the other contents are the same as in Example 1.
[0064] In this example, the obtained powdery composite probiotics are prepared into capsules for convenient consumption.
Embodiment 3
[0066] Present embodiment formula is as follows:
[0067]
[0068] The powdery bacterial strain composition is added into olive oil to prepare oil drops; the powdery bacterial strain composition: olive oil=0.01 g: 5 ml.
[0069] Olive oil can also be substituted with sunflower oil.
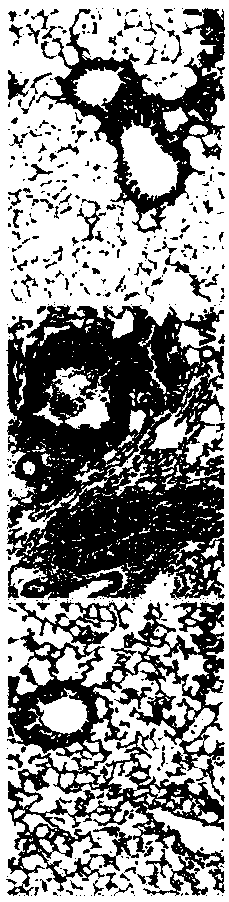
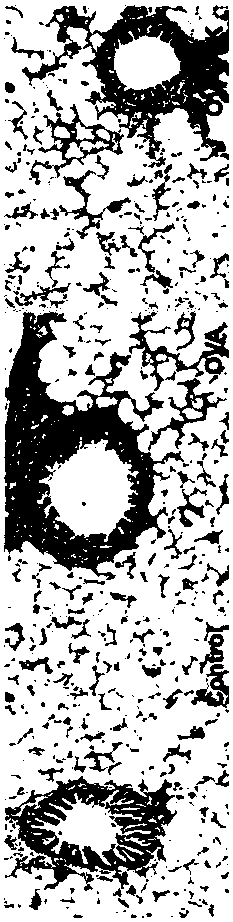
[0070] The effect of the probiotic composition prepared in Example 3 was verified.
[0071] 【Experimental animals】
[0072] The present invention is through 6~8 week SPF level (Specific pathogen Free (SPF)), BALB / c male mouse (purchased from the albino laboratory mouse of Fourth Military Medical University Experimental Animal Center) is randomly divided into 3 The control group (Control), the asthma group (OVA) and the probiotic composition intervention group (OVA+LK) were divided into two groups, 25 rats in each group. They were raised in the animal room of the Basic Department of the Fourth Military Medical University, with a constant room temperature of 21-25°C, a humidity of 50%-65%, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com