Preparation method of lithium ion battery negative electrode material zinc manganate
A technology for zinc manganate and lithium-ion batteries, applied in battery electrodes, secondary batteries, manganate/permanganate, etc., can solve poor electrochemical performance, complicated preparation process, long preparation cycle, etc. problems, to achieve excellent specific capacity, high product purity, and shortened diffusion distance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Solution preparation: When preparing the solution, weigh 0.01mol of zinc acetate and 0.02mol of manganese sulfate and dissolve them in 100mL of deionized water, weigh 0.15mol of oxalic acid and dissolve them in 100mL of absolute ethanol, and stir until completely dissolved.
[0027] (2) Solution mixing: under vigorous magnetic stirring, the aqueous solution of zinc acetate and manganese sulfate is slowly added dropwise to the ethanol solution of oxalic acid at a rate of 5 mL / min, centrifuged, washed with water and alcohol, and the precipitate is vacuum-dried. get the precursor;
[0028] (3) High temperature sintering: calcining the precursor at 600°C for 3 hours to obtain zinc manganate.
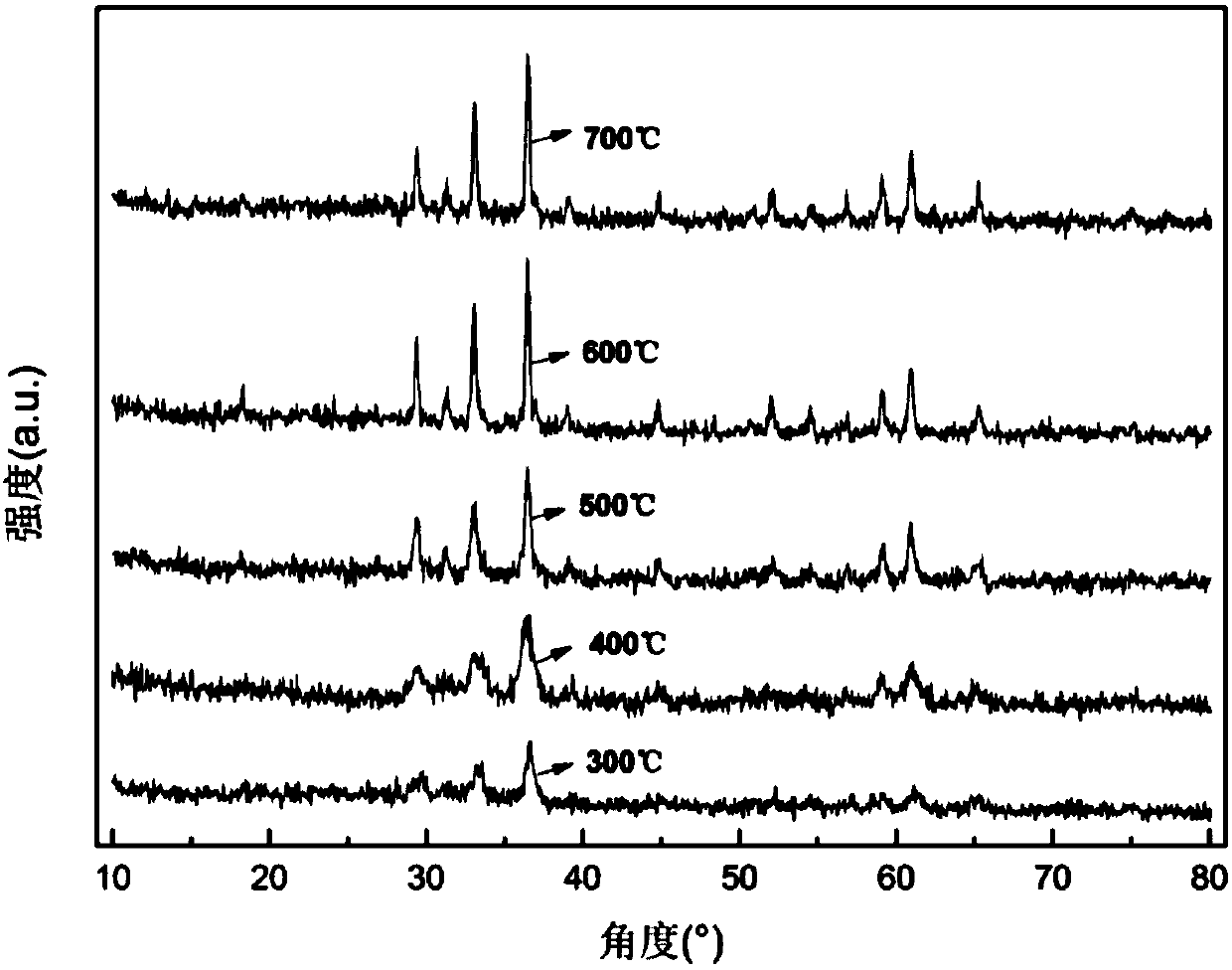
[0029] figure 1 It is the XRD pattern of zinc manganate obtained by sintering the precursor at different temperatures from 300 to 700°C. It can be seen that as the temperature continues to rise, the crystallinity increases and the average particle size increases. The degree of c...
Embodiment 2
[0036] This embodiment is basically the same as Embodiment 1, the only difference is that the rate of addition is 3mL / min. Since manganese sulfate is completely soluble in water and insoluble in ethanol, when the mixed aqueous solution of zinc acetate and manganese sulfate is added to the ethanol solution of ultra-high concentration oxalic acid, in the local area, manganese sulfate and oxalic acid complex, and this complex is easy Mature and grow up. Therefore, when the dropping rate slows down, due to the generation of by-products, the yield of the precursor decreases, resulting in a decrease in the yield of zinc manganate.
Embodiment 3
[0038] This embodiment is basically the same as Embodiment 1, the only difference is that the sintering is carried out at 300, 400, 500, 600, 700, 1000°C. figure 1 It can be seen that with the continuous increase of sintering temperature, ZnMn 2 o 4 The sharper the diffraction characteristic peak of the powder, it shows that the crystallinity is increasing continuously, but at the same time, with the increase of calcination temperature, the broadening phenomenon of the diffraction characteristic peak is weakened, indicating that high temperature sintering leads to the increase of ZnMn 2 o 4 The powder agglomeration phenomenon is aggravated, and the average particle size increases. For the screening of materials, it is necessary to comprehensively consider the influence of crystallinity and agglomeration factors, figure 1 Among them, when the calcination temperature is 600°C and 700°C, there is little difference in the diffraction characteristic peaks between the two, that i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com