Method for preparing N-(5-methyl furfuryl) p-methoxyaniline by using one-pot method
A technology for p-methoxyaniline and methyl furfuryl, which is applied in the field of compound synthesis, can solve the problems of high preparation cost of methoxyaniline, cumbersome process, low yield and the like, achieves maximum utilization of resources and simplifies operation process , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the autoclave with a magnetic stirring bar, add 10mg Pd / C, 500ul 5-methylfurfural, 20ml ethyl acetate, 0.6783g p-methoxyaniline successively, close the autoclave, and replace the air in the reactor with hydrogen , and ensured that the initial hydrogen pressure of the reaction was 1.2Mpa, the high-pressure reactor was placed in an electric heating mantle, the temperature was raised to 90°C, and the reaction was stirred for 4h. Gas chromatographic analysis was carried out after the reaction, and it was calculated by the area normalization method that the conversion rates of the substrate 5-methylfurfural and p-methoxyaniline reached 96.87% and 88.60% respectively, and the intermediate product N-(5-methoxyaniline in the system The percentages of N-(5-methylfurfuryl)-p-methoxyphenylimine and the target product N-(5-methylfurfuryl)-p-methoxyaniline were 31.04% and 55.10%, respectively.
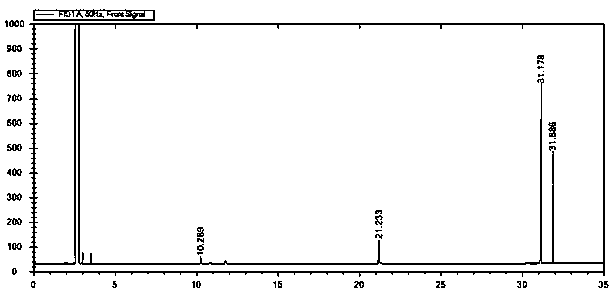
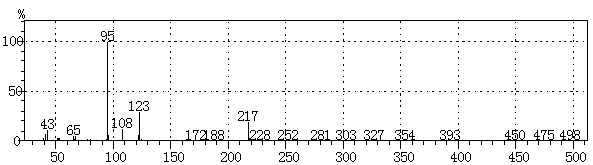
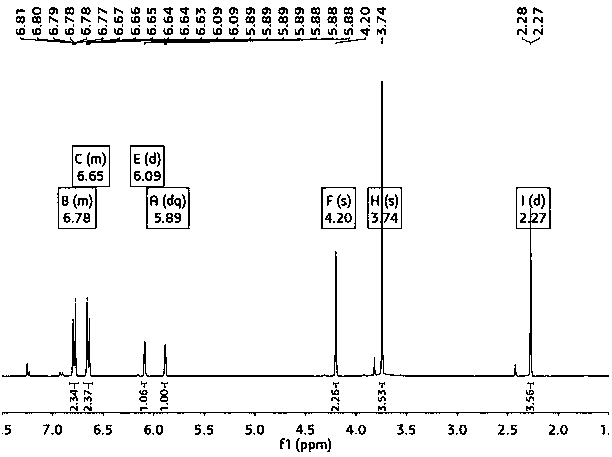
[0023] figure 1 It is the gas chromatogram of the reaction liquid obtained in the pr...
Embodiment 2
[0027] In the autoclave with a magnetic stirring bar, add 15mg Pd / C, 500ul 5-methylfurfural, 20ml ethyl acetate, 0.6781g p-methoxyaniline successively, close the autoclave, and replace the air in the reactor with hydrogen , and ensured that the initial hydrogen pressure of the reaction was 0.8Mpa, the high-pressure reactor was placed in an electric heating mantle, the temperature was raised to 70°C, and the reaction was stirred for 2h. Gas chromatographic analysis was carried out after the reaction, and calculated by the area normalization method, the conversion rates of the substrate 5-methylfurfural and p-methoxyaniline reached 94.30% and 87.22% respectively, and the intermediate product N-(5-methoxyaniline in the system The percentages of N-(5-methylfurfuryl)-p-methoxyphenylimine and the target product N-(5-methylfurfuryl)-p-methoxyaniline were 26.70% and 97.10%, respectively.
Embodiment 3
[0029] In the autoclave with magnetic stirring bar, add 10mg Pd / C, 500ul 5-methylfurfural, 20ml ethanol, 0.6779g p-methoxyaniline successively, close the autoclave, and replace the air in the reactor with hydrogen, and Ensure that the initial hydrogen pressure of the reaction is 1.2Mpa, place the high-pressure reactor in an electric heating mantle, raise the temperature to 90°C, and stir for 4 hours. Gas chromatographic analysis was carried out after the reaction, and the calculation by area normalization method showed that the conversion rates of substrate 5-methylfurfural and p-methoxyaniline reached 100% and 91.60% respectively, and the intermediate product N-(5-methoxyaniline in the system The percentages of N-(5-methylfurfuryl)-p-methoxyphenylimine and the target product N-(5-methylfurfuryl)-p-methoxyaniline were 25.44% and 66.15%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com