Application of C-type cpg as adjuvant in HBV preventive and therapeutic vaccine and preparation method thereof
A therapeutic vaccine and preventive technology, applied in the fields of biotechnology and genetic engineering vaccines, can solve the problem of weak activation of IL-12 cytokines in the NF-κB signaling pathway, and achieve the effect of facilitating development and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059]In a preferred embodiment of the present invention, the method for preparing the above-mentioned HBV preventive vaccine includes: adding aluminum hydroxide dropwise to the HBsAg solution, mixing upside down, and then adding the CpG M362 solution to obtain the HBV preventive vaccine. Vaccine, this vaccine solution can be made into a unit-dose vaccine preparation for injection after being subpackaged.
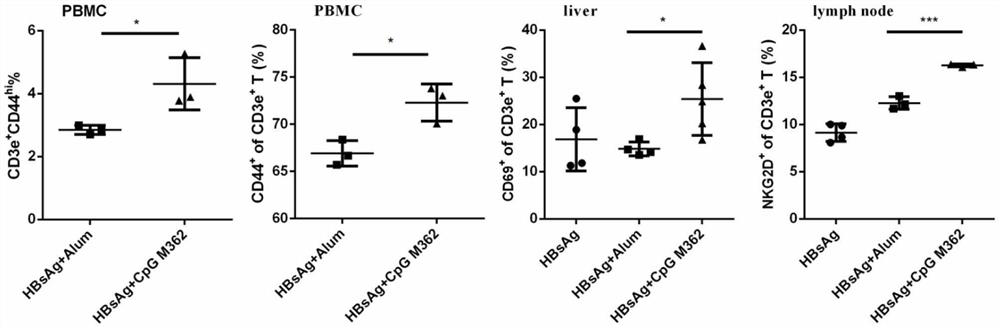
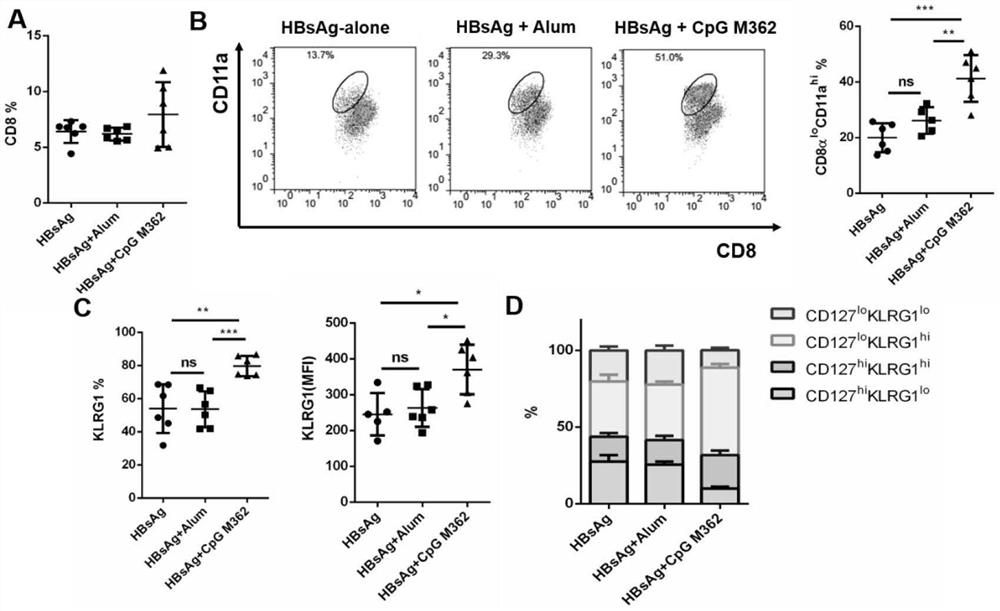
[0060]In a preferred embodiment of the present invention, the present invention also provides an application of type C CpG M362 as an adjuvant in a therapeutic vaccine for HBV.
[0061]In a preferred embodiment of the present invention, the active ingredients of the HBV therapeutic vaccine include rHBV vaccine and CpG M362.
[0062]Among them, the rHBV vaccine is a recombinant hepatitis B vaccine (Hansenula yeast) produced by Dalian Hanxin. This strain of vaccine has been used clinically for many years, can stimulate the body to produce immunity against hepatitis B virus, and can effectiv...
Embodiment 1
[0079]The preparation method of the preventive hepatitis B vaccine of the present invention:
[0080]Raw materials and sources:
[0081]HBsAg is a product derived from Hansenula yeast produced by Dalian Hanxin Biological Products Co., Ltd., with a concentration of 200μg / ml.
[0082]Alhydrogelinvivogen company, specification: 250ml, Al(OH)3Concentration: 9-10mg / ml
[0083]CpG M362, invivogen company, specification: 1mg. Prepare 1mg / ml with normal saline for injection, filter and sterilize with 200μm sterile filter membrane, and store frozen at -20°C.
[0084]Preparation method of vaccine:
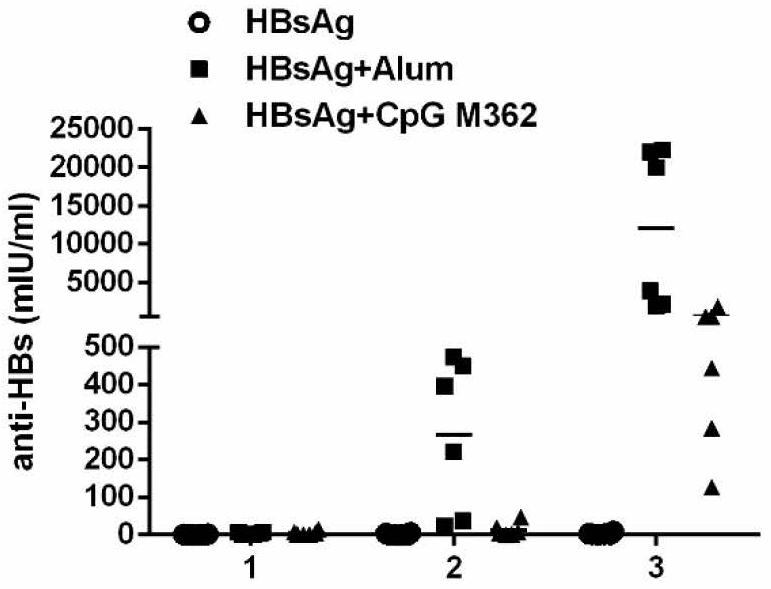
[0085]Take 100μl of HBsAg in a sterile Ep tube, add 700μl of saline, and mix well; then take 100μl of Alhydrogel2%, drop it into the HBsAg solution, mix upside down for 5min; finally add 100μl of CpG M362 solution. The prepared vaccine solution contains 20μg of hepatitis B surface antigen, Al(OH) per ml3100μg, CpG M362 100μg.
[0086]Immunization strategies for preventive hepatitis B vaccine:
[0087]The experimental gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com