Sun-proof emerald pyridinone reactive dye compound as well as preparation method and application thereof
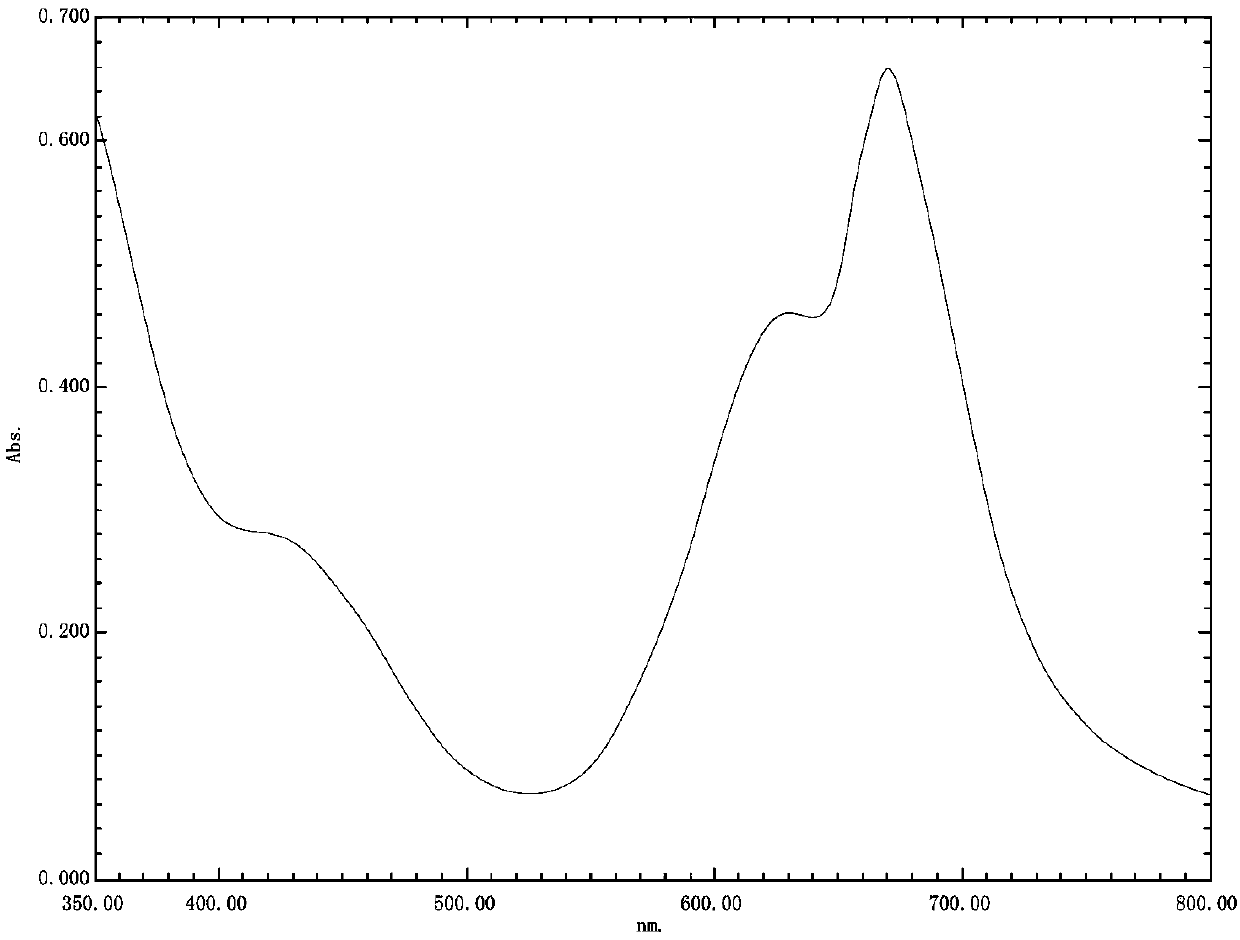
A technology of reactive dyes and pyridone, applied in the field of dyes and their preparation, can solve the problems of poor diffusion performance, solubility difference, poor compatibility, etc., achieve excellent light fastness, improve light fastness, and develop well potential effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
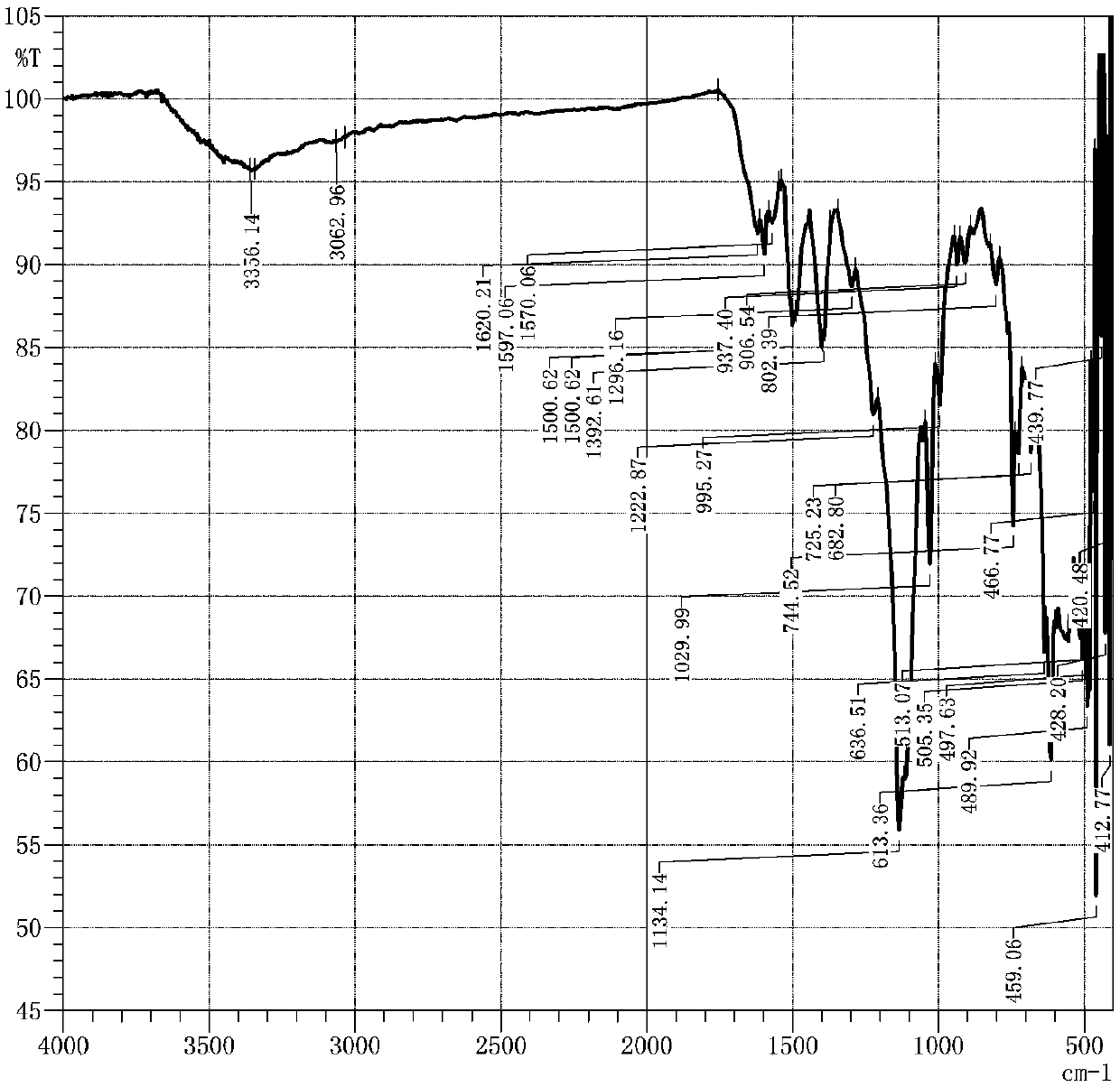
[0044] This embodiment discloses a sunfast emerald green pyridone reactive dye compound and a preparation method thereof, the specific steps are as follows:
[0045] (a) Chlorosulfonation reaction: at 40°C, slowly (within half an hour) add 0.100mol of copper phthalocyanine powder to 220mL of chlorosulfonic acid, stir for 30min, then raise the temperature to 135-140°C, maintain the reaction for 3h; cool down to 80°C, and dropwise added 0.504mol thionyl chloride within 2h, then raised the temperature to 90°C, and kept the temperature for 2h; after the reaction was completed, it was lowered to room temperature, poured into ice water while stirring, and precipitated; stood still, filtered, The filter cake was washed with ice water until neutral to obtain the copper phthalocyaninesulfonyl chloride filter cake, which was set aside;
[0046] (b) Primary condensation reaction: 0.101mol cyanuric chloride and 60g crushed ice were beaten for 30 minutes at 0°C; 0.100mol 2,4-diaminobenzene...
Embodiment 2~9
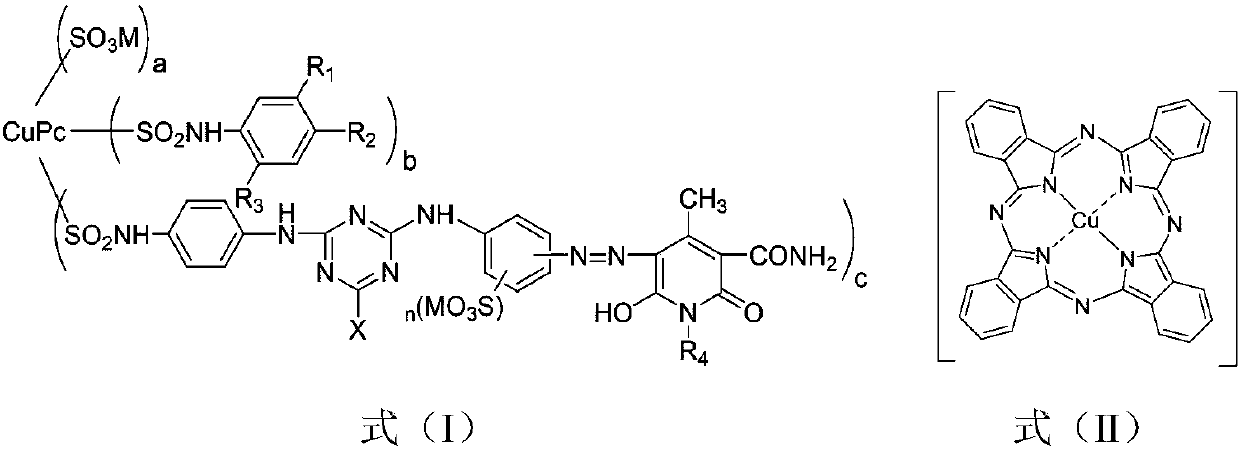
[0052] The examples respectively disclose a sunfast emerald green pyridone reactive dye compound and a preparation method thereof, and the specific steps are similar to Example 1. The difference is: the 4-(β-sulfate group ethyl sulfone group) aniline in step (f) is changed into 4-(β-sulfate group ethyl sulfone group) aniline-2-sulfonic acid, 2-methoxy Base-4-(β-sulfate ethylsulfone)aniline, 2,5-dimethoxy-4-(β-sulfate ethylsulfone)aniline, 2-amino-5-(β- Sulfateethylsulfone)benzoic acid, 3-(β-sulfateethylsulfone)aniline, 3-(β-sulfateethylsulfone)aniline-6-sulfonic acid, 2-methoxy Base-5-(β-sulfate group ethyl sulfone group) aniline and 2-hydroxyl-5-(β-sulfate group ethyl sulfone group) aniline, consumption is 0.100mol; Through step (f), finally obtain The emerald green pyridone reactive dye compound containing copper phthalocyanine and pyridone dichromate has the structures shown in formula (I-2A) to formula (I-9A) in Table 1.
Embodiment 10~18
[0054] The examples respectively disclose a light-fast emerald green pyridone reactive dye compound and a preparation method thereof, and the specific steps are similar to those in Examples 1 to 9. The difference is: the 2,4-diaminobenzenesulfonic acid in the step (b) is changed to m-phenylenediamine-4,6-bissulfonic acid, and the dosage is 0.100mol; after the step (f), the copper-containing phthalein The emerald green pyridone reactive dye compound of cyanine and pyridone dichromophore has the structure shown in the formula (I-10A) ~ formula (I-18A) in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com