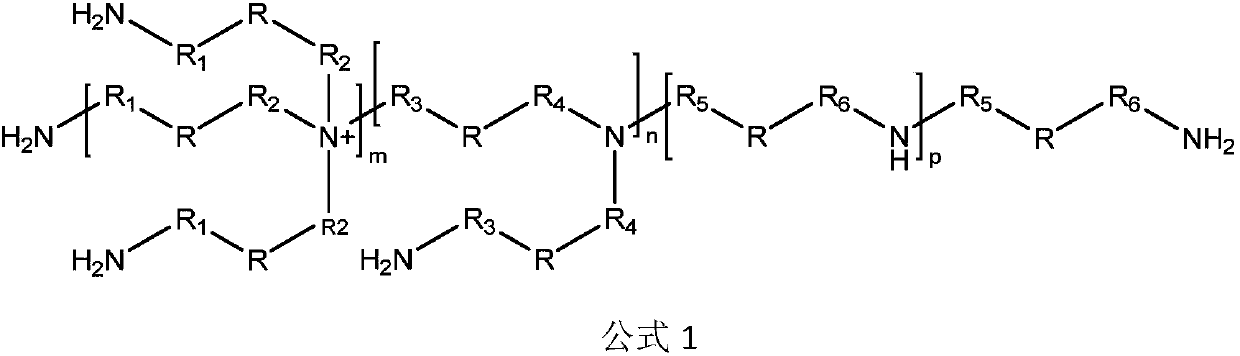
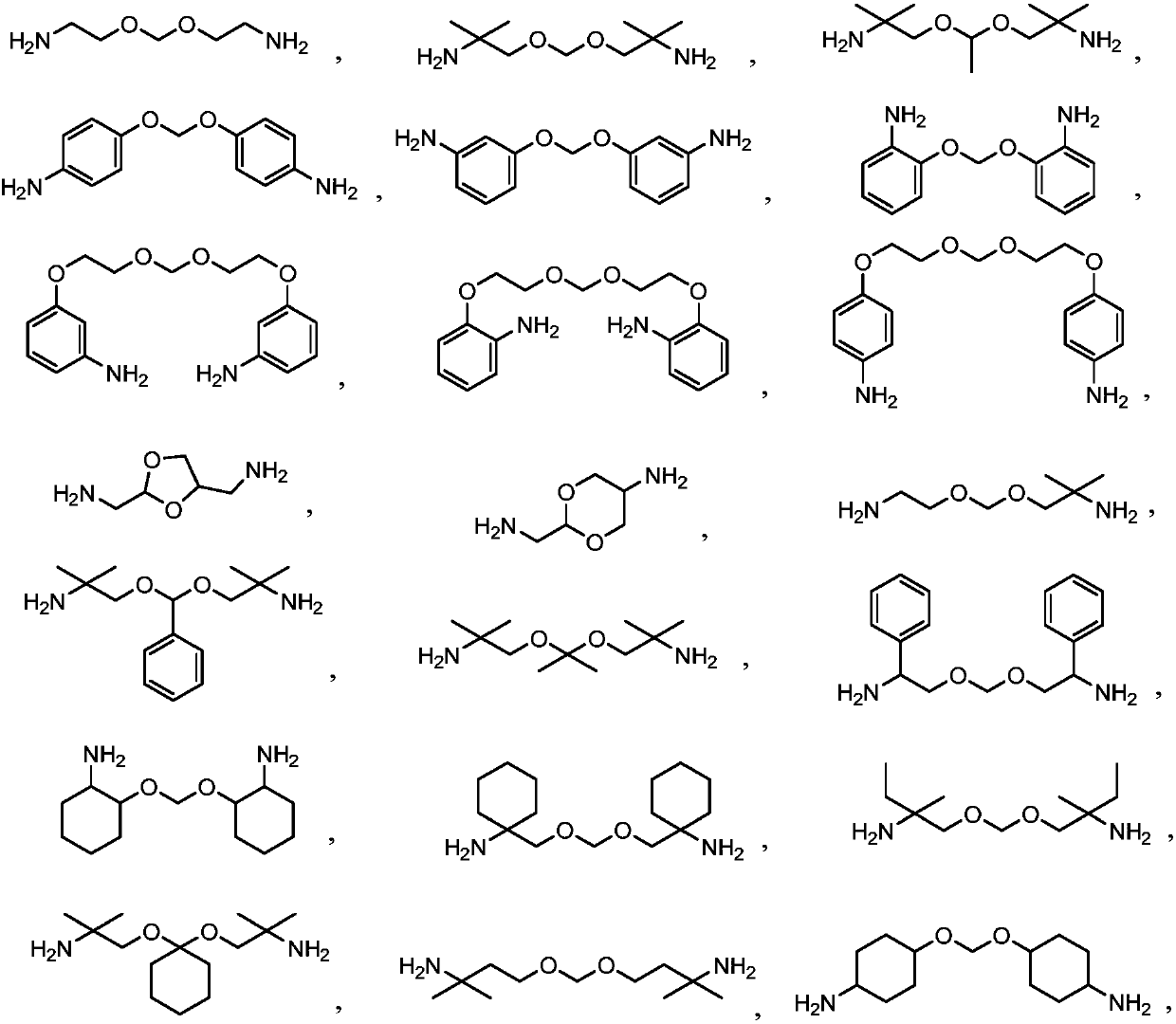
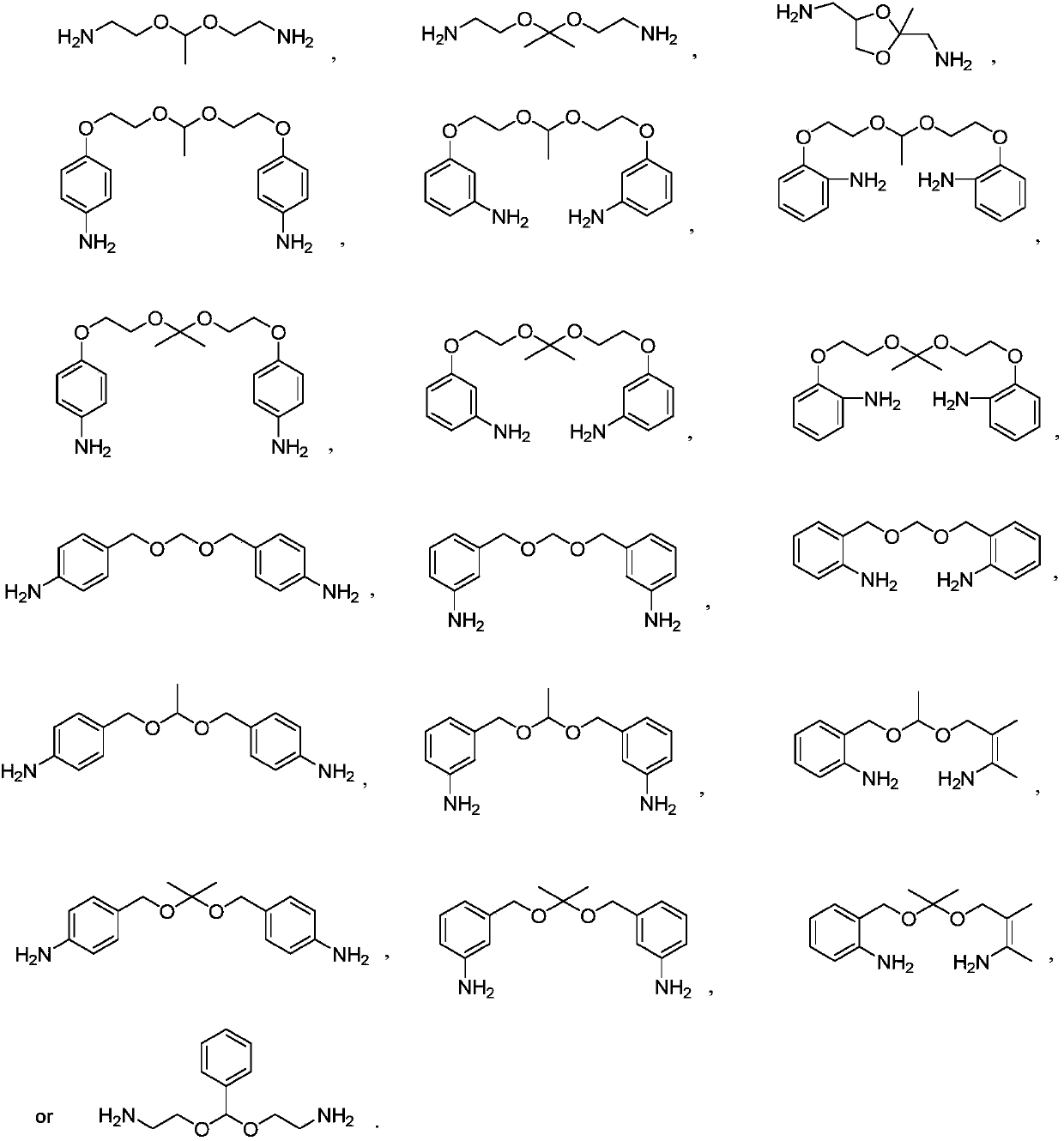
Degradable polyurethanes and composites thereof
A polyurethane and polyester diol technology, applied in the field of isocyanate resin composition, can solve the problems of low commercialization, pollution, and immaturity of degradable polyurethane technology, and achieve excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the synthesis of crosslinking agent I
[0071] Put 296 grams of ammonia solution into the reaction bottle, start stirring, add 32 grams of ammonium chloride, stir to dissolve, then add 50 grams of bis(2-chloroethoxy)methane at room temperature, then heat up to 80 ° C, and stir the reaction After 6 hours, TLC detects the end point of the reaction. After the reaction is completed, most of the solution is concentrated under reduced pressure, and the residual solution is transferred to a reaction bottle. and 300 milliliters of mixed solvents of ethanol (volume ratio 3:1), extract the aqueous phase 3 times, combine the organic phases, dry the organic phases with anhydrous sodium sulfate, filter, wash the filter cake once with a small amount of solvent, and concentrate the filtrate to dryness , to obtain about 20 grams of crosslinking agent I, its total amine value is 5.9 mmol / g.
Embodiment 2
[0072] Embodiment 2: the synthesis of cross-linking agent II
[0073] Put 69.4 grams of ethylenediamine into the reaction bottle, start stirring, add 10 grams of bis(2-chloroethoxy)methane dropwise at room temperature, drop it in about 1 hour, then raise the temperature to 120°C, and stir for 20 hours , TLC detects the end point of the reaction, after the reaction is over, concentrate most of the solution under reduced pressure, transfer the residual solution to the reaction bottle, adjust the pH ≥ 10 with 30% sodium hydroxide solution below 25 ° C, and then use 90 ml of di Extract the aqueous phase 3 times with methyl chloride, combine the organic phases, dry the organic phases with anhydrous sodium sulfate, filter, wash the filter cake once with a small amount of solvent, and concentrate the filtrate to dryness to obtain about 12 grams of crosslinking agent II, whose total amine value 9.7 mmol / g.
Embodiment 3
[0074] Embodiment 3: the synthesis of cross-linking agent III
[0075] Put 500 grams of ammonia solution into the reaction bottle, start stirring, add 1 gram of urotropine, stir to dissolve, then add 50 grams of bis(2‐chloroethoxy)methane at room temperature, then heat up to 80 ° C, stir React for 6 hours, TLC detects the end point of the reaction. After the reaction is over, concentrate most of the solution under reduced pressure, transfer the residual solution to the reaction bottle, and adjust the pH ≥ 10 with 30% sodium hydroxide solution below 25 ° C, and then use 300 milliliters of mixed solvents of chloroform and ethanol (volume ratio 3:1), extract the aqueous phase in 3 times, combine the organic phases, and dry the organic phases with anhydrous sodium sulfate, filter, and the filter cake is washed once with a small amount of solvent, and the filtrate is concentrated to Dry, obtain about 14 grams of crosslinking agent III, its total amine value is 5.2 mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com