A polyolefin reactive telechelic pre-polymer
A prepolymer, polyolefin technology, applied in the field of manufacturing polyolefin reactive telechelic prepolymer, can solve the problems of weakened mechanical properties, poor functionalization position and quantity control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0038] The following examples illustrate the invention but are not intended to limit the scope of the invention.
[0039] Chain transfer agent (CTA) synthesis
[0040] Using Biochimica et Biophysica Acta (BBA); He et al., 1995, vol. 1253, p. 117 and Macromolecules Nagarkar, et al. 2012, vol. 45, p. 4447 A tert-butoxycarbonyl-protected amino chain transfer agent is made by the method described in ; and is shown in Reaction Scheme 1 below:
[0041]
[0042] plan 1
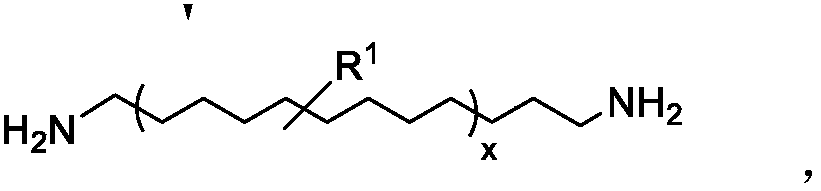
[0043] Specifically, the bromine in 1,4-dibromo-2-butene was replaced by phthalimide in a nucleophilic attack to give compound 1; removal of this group under acidic conditions gave compound 2, Di-hydrochloride salt of diamino compound. Derivatives of this di-amino compound are then protected with tert-butoxycarbonyl to obtain compound 3, but-2-ene-1,4-diyl(E)-di-tert-butyl dicarbamate.
[0044] As shown in Scheme 2 below, di-tert-butyl but-2-ene-1,4-diyl(E)-dicarbamate was then contacted as chain transfer agen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com