Triple inactivated vaccine for porcine epidemic diarrhea, swine transmissible gastroenteritis and porcine delta coronavirus and preparation method of triple inactivated vaccine
A technology for porcine epidemic diarrhea and inactivated vaccines, which is applied in the field of animal multiple inactivated vaccines and its preparation, can solve the problems of pig industry losses, lack of effective treatment measures and vaccines, etc., and achieves reduced epidemic prevention costs and simple immunization methods , the effect of simplifying the immunization procedure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The source of embodiment 1 strain
[0031] 1. Porcine epidemic diarrhea virus PEDV-KB2013-4 strain
[0032] The disease material collected from a pig farm and tested positive for porcine epidemic diarrhea antigen by RT-PCR was added to PBS at a ratio of 1:10 (weight: volume). Filter twice with a μm filter, and store the filtrate below -20°C for future use.
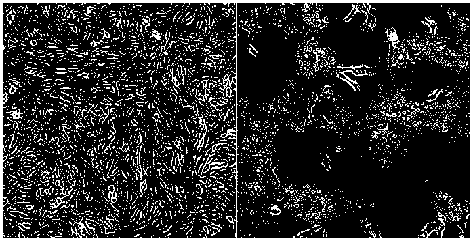
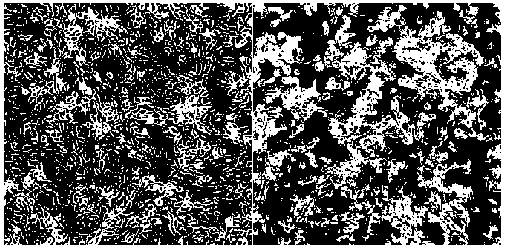
[0033] The Vero cells covered with a dense monolayer were inoculated with the filtrate at a dose of 10%, and after adsorption, the maintenance solution containing trypsin was added, and cultured at 37°C. At the same time, blank cells were set as a negative control. Obvious cytopathic changes in the second passage ( figure 1 ), the lesions were basically stable after the fifth passage.
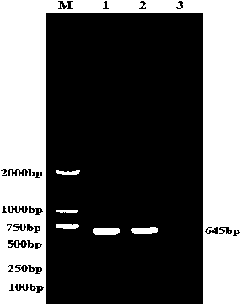
[0034] Using Primer Premier 5.0 gene analysis software, refer to the PEDV M protein gene in Genbank to design detection primer pairs: upstream primer PEDV-F: 5'-AACGGTTCTATTCCCGTTGATG-3'; downstream primer PEDV-R: 5'-TAAATGAAGCACT...
Embodiment 2
[0050] The characteristic of embodiment 2 virus strains
[0051] 1. Specificity
[0052] Dilute porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine deltacoronavirus to 200 TCID respectively with MEM medium 50 / 0.1ml, mixed with the corresponding amount of PEDV, TGEV, PDCoV-specific positive serum, neutralized at 37°C for 1 hour, and respectively inoculated into 96-well cells of Vero cells, ST cells, and ST cells that had grown into a good monolayer Culture plate, inoculate 4 wells, 200μl / well, set normal cell control and unneutralized virus control at the same time, set at 37°C, containing 5% CO 2 The cells were cultured in an incubator for 7 days, and the cytopathic changes were observed and recorded daily. Neither the positive serum neutralization group nor the normal cell control group had cytopathic changes; all the unneutralized virus control groups had cytopathic changes.
[0053] 2. Virulence
[0054] A total of 15 healthy suscep...
Embodiment 3
[0057] Example 3 Preparation and Inspection of Triple Inactivated Vaccine of Porcine Epidemic Diarrhea, Porcine Transmissible Gastroenteritis and Porcine Delta Coronavirus
[0058] 1. Materials and methods
[0059] 1.1 cells
[0060] Vero cells and ST cells were purchased from China Veterinary Drug Administration.
[0061] 1.2 Preparation of virus liquid for seedling production
[0062] Preparation of Porcine Epidemic Diarrhea Virus Seeds: The virus was prepared by the spinner bottle cell culture method. Inoculate Vero cells covered with dense monolayer with 0.2% dose of qualified porcine epidemic diarrhea virus seed virus, and add MEM with a final concentration of 10 μg / mL of trypsin, 100 U / ml of penicillin, and 100 μg / ml of streptomycin The maintenance solution was cultured at 37°C for 48-72 hours. When the cytopathic effect reached more than 80%, the virus liquid was harvested, frozen and thawed twice, and kept below -20°C for later use.
[0063] Preparation of Porcine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com