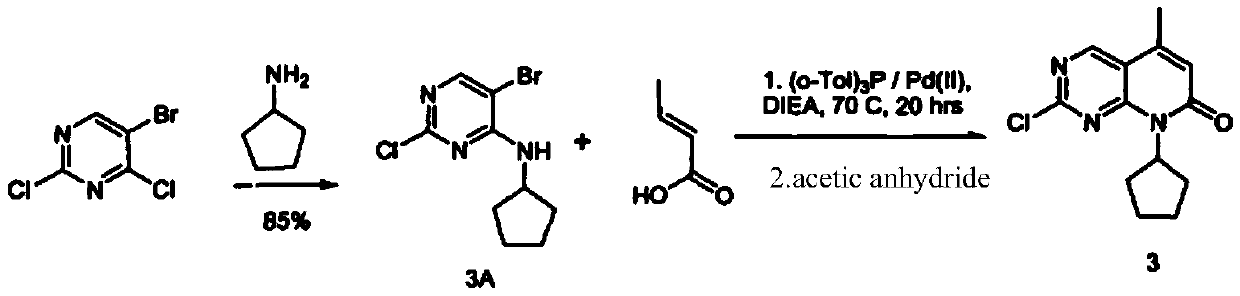
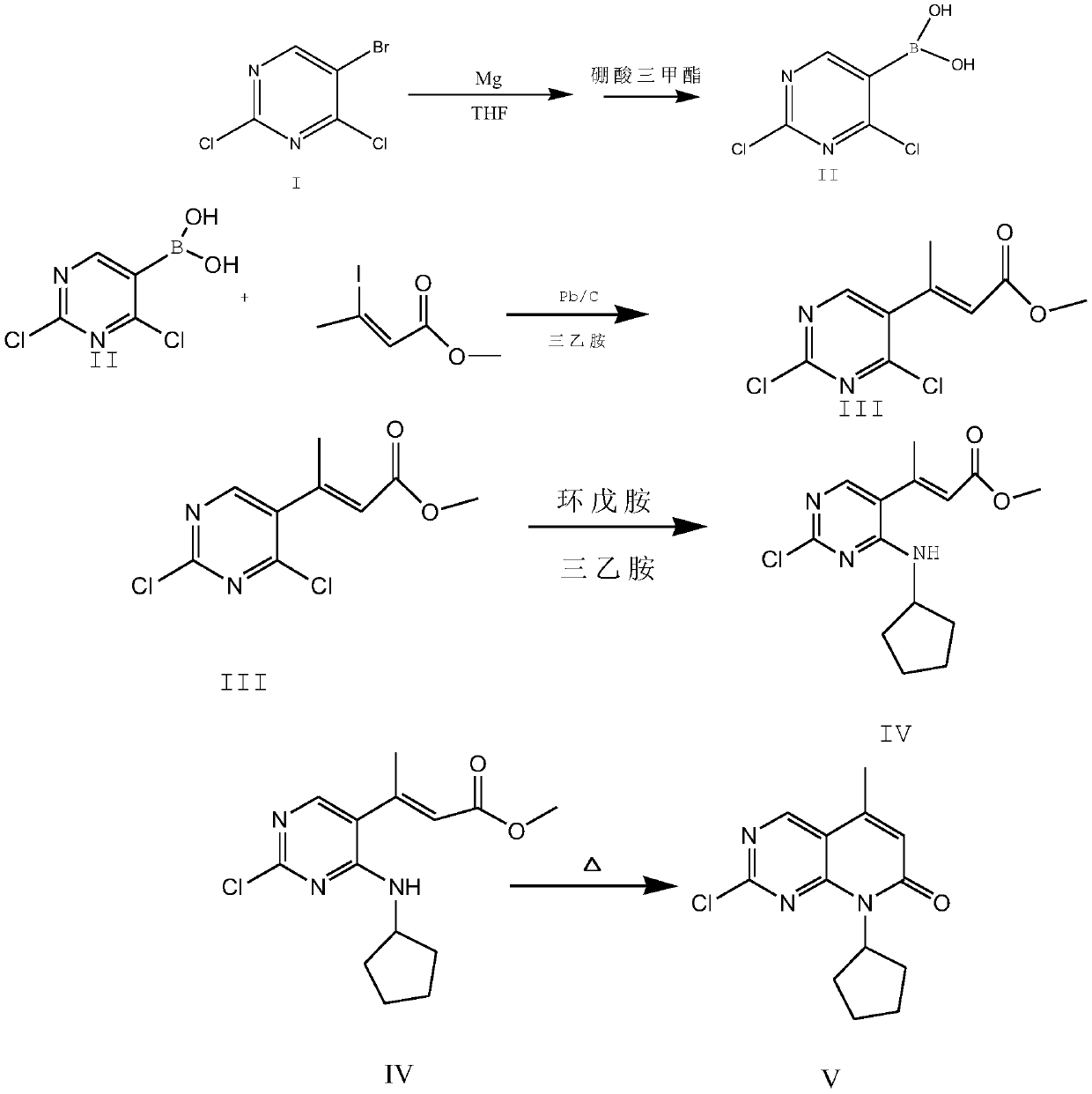
A kind of synthetic method of palbociclib intermediate
A synthetic method and intermediate technology, applied in the field of palbociclib intermediates, can solve the problems of complex steps, unsuitability for industrial production, and low product yield, and achieve simple operation, high product yield, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Preparation of compound II
[0029] Dissolve 341.7 g of compound I into 1800 g of tetrahydrofuran in two steps, that is, the first time is 150 g of compound I+800 g of tetrahydrofuran, and the second is 191.7 g of compound I+1000 g of tetrahydrofuran.
[0030] 1800g of tetrahydrofuran was added to the reaction flask, 45g of magnesium scraps were put in, 1 iodine was added, 150g of the tetrahydrofuran solution of compound I was added dropwise to the Grignard flask under nitrogen protection, the temperature was raised to 30°C, and the remaining compounds were added after the reaction was initiated. The tetrahydrofuran solution of I was added dropwise to the Grignard reaction flask at a controlled temperature of 25-32°C, and continued stirring at this temperature for 2 hours after dropping. Control the temperature to -10-0°C, add 165g of trimethyl borate dropwise to the Grignard reaction flask, complete the dropwise addition and react for 30 minutes, control the materia...
Embodiment 2
[0035] 1) Preparation of compound II
[0036] 341.7 g of compound I was dissolved in 1500 g of tetrahydrofuran in two times, that is, the first time was 150 g of compound I+600 g of tetrahydrofuran, and the second time was 191.7 g of compound I+900 g of tetrahydrofuran.
[0037] 1500g of tetrahydrofuran was added to the reaction flask, 42g of magnesium scraps were put in, 1 iodine was added, 150g of the tetrahydrofuran solution of compound I was added dropwise to the Grignard flask under nitrogen protection, the temperature was raised to 30°C, and the remaining compounds were added after the reaction was initiated. The tetrahydrofuran solution of I was added dropwise to the Grignard reaction flask at a temperature of 25-32°C, and continued stirring at this temperature for 2.5 hours after dropping. Control the temperature to -10-0°C, add 163g of trimethyl borate into the Grignard reaction flask, dropwise and react for 35 minutes, control the material to 0-10°C, add it to 12kg o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com