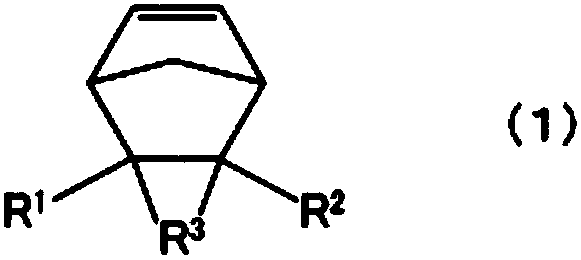
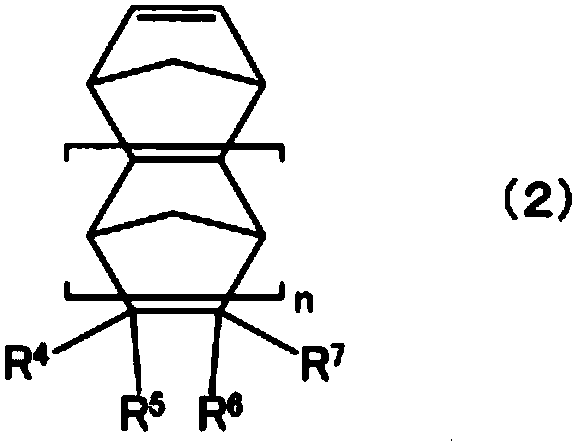
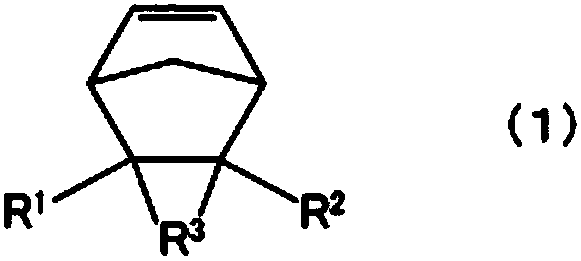
Cyclopentene ring-opened copolymer
A kind of technology of cyclopentene and copolymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] (Manufacture of cyclopentene ring-opening copolymer)
[0135] Under nitrogen environment, add 541 parts of cyclopentene, 163 parts of 1,4-endomethylene-1,4,4a,9a-tetrahydro-9H-fluorene (MTHF ), 2800 parts of toluene and 0.61 parts of 1-hexene, to which 0.074 parts of (1,3-dicycloimidazolidin-2-ylidene) (tricyclohexylphosphine)benzylidene ruthenium dichloride was dissolved Polymerization was carried out at 25°C for 4 hours in a polymerization catalyst solution in 30 parts of toluene. After 4 hours of polymerization, in the pressure-resistant glass reaction vessel, add excess isopropanol to terminate the polymerization, inject the solution in the pressure-resistant glass reaction vessel into a solution containing 2,6-di-tert-butyl p-cresol (BHT ) in a large excess of isopropanol. Next, the precipitated polymer was recovered, washed with isopropanol, and vacuum-dried at 40° C. for 3 days to obtain 320 parts of a cyclopentene / MTHF ring-opened copolymer. For the cyclopent...
Embodiment 2
[0139] The amount of cyclopentene was changed to 193 parts, the amount of 1,4-methano-1,4,4a,9a-tetrahydro-9H-fluorene (MTHF) was changed to 85 parts, and the amount of toluene The usage amount was changed to 1100 parts, the usage amount of (1,3-dicycloimidazolidin-2-ylidene)(tricyclohexylphosphine)benzylidene ruthenium dichloride was changed to 0.028 parts, and 0.61 parts were replaced 1-hexene and 0.47 parts of allyltriethoxysilane were used, and the polymerization reaction was carried out in the same manner as in Example 1 to obtain 178 parts of polymer chains with triethoxysilane at the end of one side. End-modified cyclopentene / MTHF ring-opening copolymer. Various measurements were performed in the same manner as in Example 1 for the obtained terminal-modified cyclopentene / MTHF ring-opened copolymer. The results are shown in Table 1. In addition, the introduction rate of the oxysilyl group in the terminal-modified cyclopentene / MTHF ring-opening copolymer was 45%.
[01...
Embodiment 3
[0142] The usage amount of cyclopentene is changed to 166 parts, the usage amount of toluene is changed to 1057 parts, (1,3-dicycloimidazolidine-2-ylidene) (tricyclohexylphosphine) benzylidene di The amount of ruthenium chloride used was changed to 0.028 parts, the amount of 1-hexene used was changed to 0.42 parts, and 163 parts of 1,4-endomethylene-1,4,4a,9a-tetrahydro-9H- Fluorene (MTHF) while using 162 parts of 70% by weight of dicyclopentadiene (DCPD) / cyclohexane solution, in addition to carrying out the polymerization reaction in the same manner as in Example 1, to obtain 177 parts of cyclopentene / DCPD ring-opening copolymers. Various measurements were performed in the same manner as in Example 1 for the obtained cyclopentene / DCPD ring-opened copolymer. The results are shown in Table 1.
[0143] Then, instead of 100 parts of the cyclopentene / MTHF ring-opening copolymer obtained in Example 1, 100 parts of the cyclopentene / DCPD ring-opening copolymer obtained were used, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com