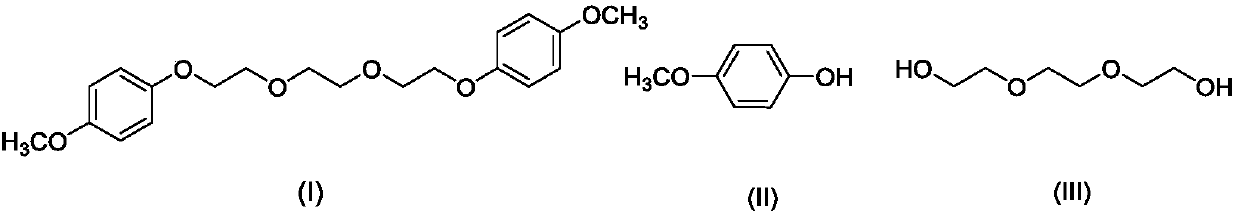
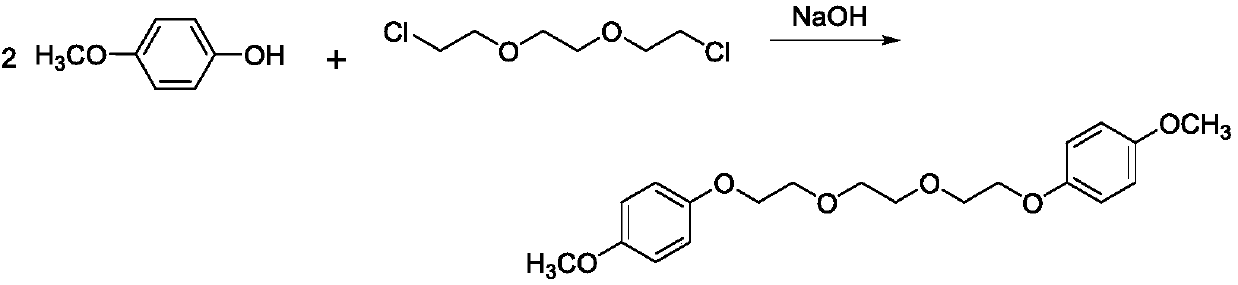
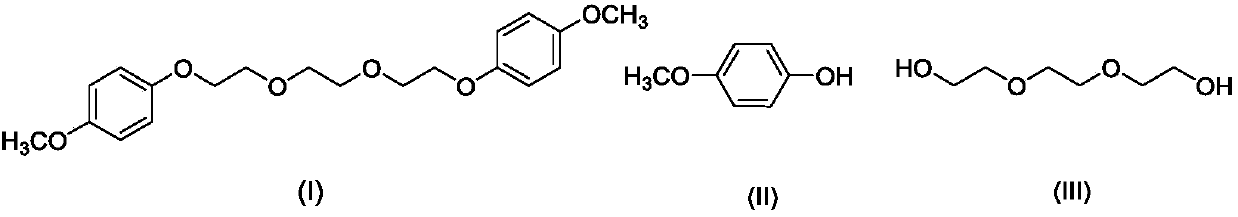
Preparation method of 1, 2-bis (2-(4-methoxyphenoxy)ethoxy)ethane
A kind of technology of methoxyphenoxy and methoxyphenol, applied in 1 field to achieve the effect of improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under stirring at a temperature of 20°C, 13.66g (110mmol) of 4-methoxyphenol and 7.51g (50mmol) of triethylene glycol were added to 100ml of acetonitrile, and 24.72g (120mmol) of dicyclohexylcarbodiimide was added thereto. ), the temperature was raised to 85° C., and after 24 hours of reaction, the acetonitrile was distilled off under reduced pressure to obtain an orange-yellow turbid liquid 1;
[0024] Extract the above orange turbid liquid 1 with 50ml dichloromethane three times, wash three times with 50ml 1M sodium hydroxide solution, wash twice with 50ml saturated sodium chloride solution (20°C), dry over anhydrous magnesium sulfate, Dichloromethane was evaporated to dryness under normal pressure to obtain a yellow oily liquid;
[0025] Add 25ml of diethyl ether to the above yellow oily liquid, raise the temperature to 35°C, filter off the insoluble matter, cool to -15°C, cool down for 3h, filter the solution to obtain 17.86g of white powdery solid 1,2-bis(2-(4-meth...
Embodiment 2
[0032] Under stirring at a temperature of 20°C, 13.66g (110mmol) of 4-methoxyphenol and 7.51g (50mmol) of triethylene glycol were added to 100ml of acetonitrile, and 24.72g (120mmol) of dicyclohexylcarbodiimide was added thereto. ), the temperature was raised to 85° C., and after 16 hours of reaction, the acetonitrile was distilled off under reduced pressure to obtain an orange-yellow turbid liquid 1;
[0033] Extract the above orange turbid liquid 1 with 50ml dichloromethane three times, wash three times with 50ml 1M sodium hydroxide solution, wash twice with 50ml saturated sodium chloride solution (20°C), dry over anhydrous magnesium sulfate, Dichloromethane was evaporated to dryness under normal pressure to obtain a yellow oily liquid;
[0034] Add 25ml of diethyl ether to the above yellow oily liquid, raise the temperature to 35°C, filter off the insoluble matter, cool to -15°C, cool down for 3h, filter the solution to obtain 17.02g of white powdery solid 1,2-bis(2-(4-meth...
Embodiment 3
[0036] Under stirring at a temperature of 20°C, 13.66g (110mmol) of 4-methoxyphenol and 7.51g (50mmol) of triethylene glycol were added to 100ml of acetonitrile, and 24.72g (120mmol) of dicyclohexylcarbodiimide was added thereto. ), the temperature was raised to 85° C., and after 30 hours of reaction, the acetonitrile was distilled off under reduced pressure to obtain an orange-yellow turbid liquid 1;
[0037] Extract the above orange turbid liquid 1 with 50ml dichloromethane three times, wash twice with 50ml 1M sodium hydroxide solution, then wash twice with 50ml saturated sodium chloride solution (20°C), dry over anhydrous magnesium sulfate, Dichloromethane was evaporated to dryness under normal pressure to obtain a yellow oily liquid;
[0038] Add 25ml of diethyl ether to the above yellow oily liquid, raise the temperature to 35°C, filter off the insoluble matter, cool to -15°C, cool down for 3h, and filter the solution to obtain 17.94g of white powdery solid 1,2-bis(2-(4-met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com