Iridium complex with double-emission properties, method for preparing iridium complex and application thereof
An iridium complex, dual emission technology, applied in indium organic compounds, platinum group organic compounds, compounds containing elements of the 8/9/10/18 group of the periodic table, etc., can solve problems such as human injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
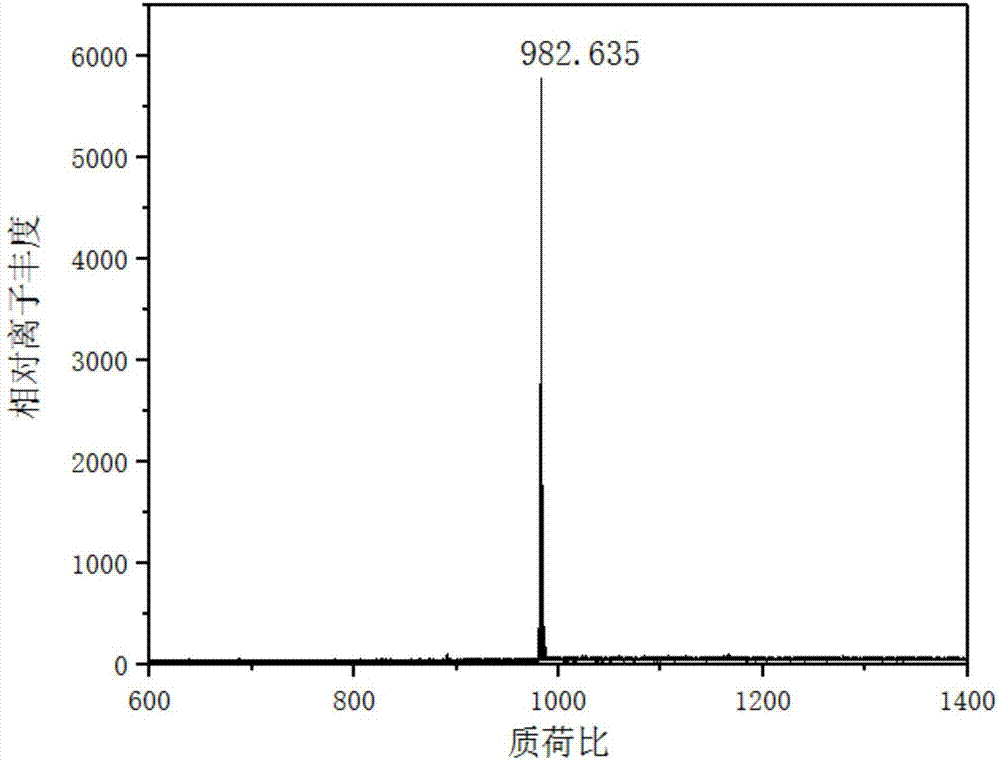
[0033] When N^N ligands are , the preparation of iridium complex Ir2, the specific steps are as follows:
[0034]
[0035] Synthesis of compound a: First, 2-aminobenzenethiol (4g, 32mmol) and p-hydroxybenzaldehyde (4.12mL) were added to a 250mL two-necked flask, and vacuum-filled with nitrogen-vacuumized on the double-row tube , loop three times. Second, redistilled N,N dimethylformamide (5 mL) was injected into the reaction system, and the reaction system was protected with nitrogen. Finally, the temperature of the reaction system was raised to 110° C., and the reaction was stirred for 72 hours. After the reaction was complete, the solvent was removed under reduced pressure, and recrystallized from ethanol to obtain a gray solid, namely compound a. Yield: 41.8%.
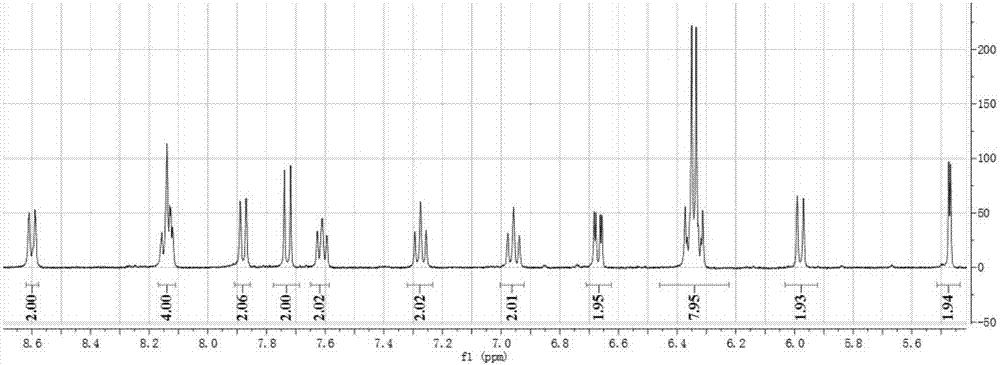
[0036] 1 H NMR (400MHz, d 6 -DMSO): δ=10.21(s,1H),8.07(d,J=7.9Hz,1H),8.00–7.87(m,3H),7.53–7.45(m,1H),7.43–7.33(m,1H ),6.97–6.88(m,2H).
[0037] Synthesis of compound b: Compound a (1.358g, 5mmol), p-fluo...
example 2
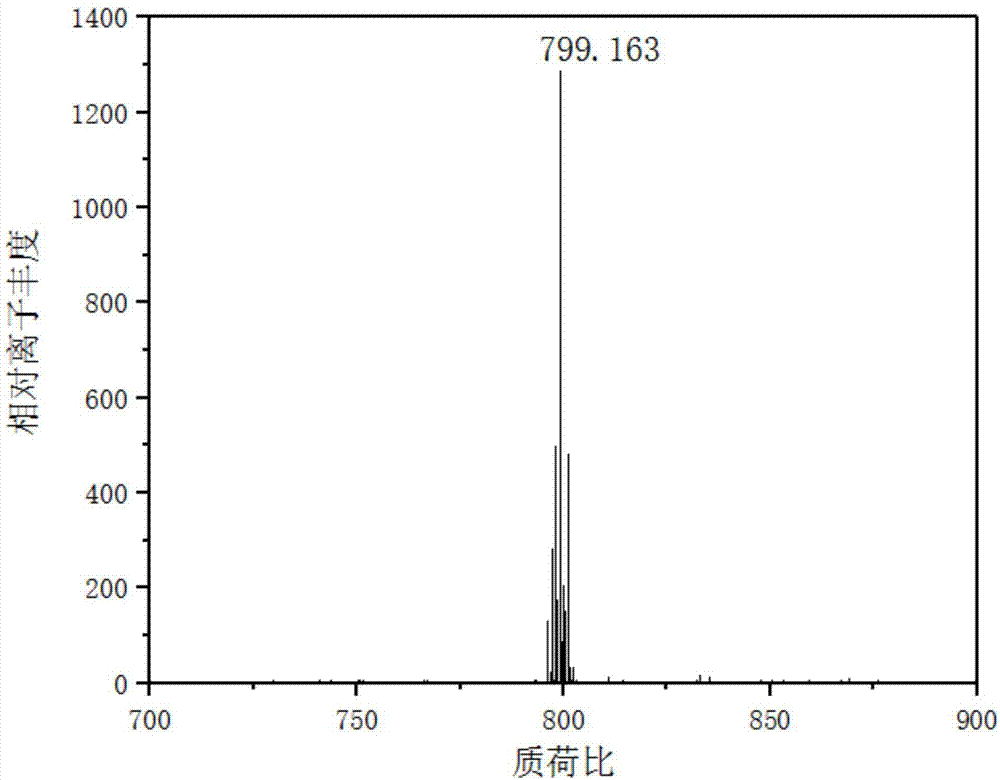
[0053] Example 2: When the N^N ligand is , the preparation of iridium complex Ir4, the synthetic steps are as follows:
[0054]
[0055] Synthesis of compound a: First, 2-aminobenzenethiol (4g, 32mmol), p-hydroxybenzaldehyde (4.12mL), was added to a 250mL two-necked flask, and vacuum-filled with nitrogen-vacuumized on the double-row tube , loop three times. Second, redistilled N,N dimethylformamide (5 mL) was injected into the reaction system, and the reaction system was protected with nitrogen. Finally, the temperature of the reaction system was raised to 110° C., and the reaction was stirred for 72 hours. After the reaction was complete, the solvent was removed under reduced pressure, and recrystallized from ethanol to obtain a gray solid, namely compound a. Yield: 41.8%.
[0056] 1 H NMR (400MHz, d 6 -DMSO): δ=10.21(s,1H),8.07(d,J=7.9Hz,1H),8.00–7.87(m,3H),7.53–7.45(m,1H),7.43–7.33(m,1H ),6.97–6.88(m,2H).
[0057] Synthesis of compound b: Compound a (1.358g, 5mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com