Structures and synthesis methods of flavone-based compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
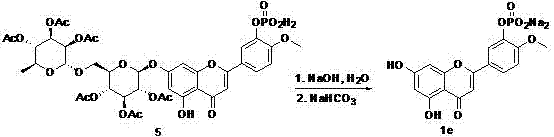
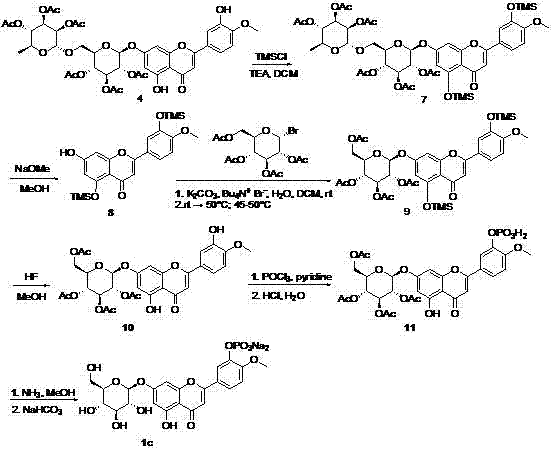
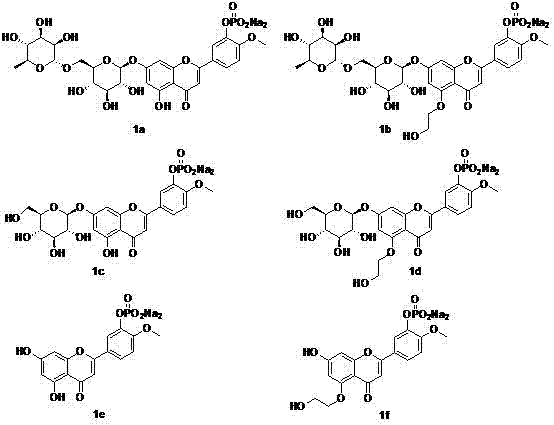
Image
Examples
Embodiment Construction
[0010] 1. Target molecule 1a:
[0011]
[0012] Synthetic steps:
[0013] 1) Add 30.4 g (50.0 mmol) of 3',5,7-trihydroxy-4'-methoxyflavone (2) and 4.2 g (50.0 mmol) of sodium acetate into 200.0 mL of acetic anhydride and heat to reflux. The initial reaction solution was a suspension, which was completely dissolved after continued heating, and the reaction was complete under reflux for 2 hours; the acetic anhydride was distilled off under reduced pressure, and 200.0 mL of distilled water was added to the residue, extracted with dichloromethane (100.0 mL×3), saturated Wash with sodium carbonate solution (50.0mL×2), wash with water until neutral; combine the aqueous phases, reverse-phase extraction with dichloromethane (50.0mL×3); combine the organic phases, remove the solvent to obtain a light yellow solid, and recrystallize from isopropanol 42.9 g of light yellow solid compound (3) was obtained with a yield of 90.8%. Ms (m / z): 945.2; NMR (400MHz,DMSO): 7.42-7.38 (m, 2H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com