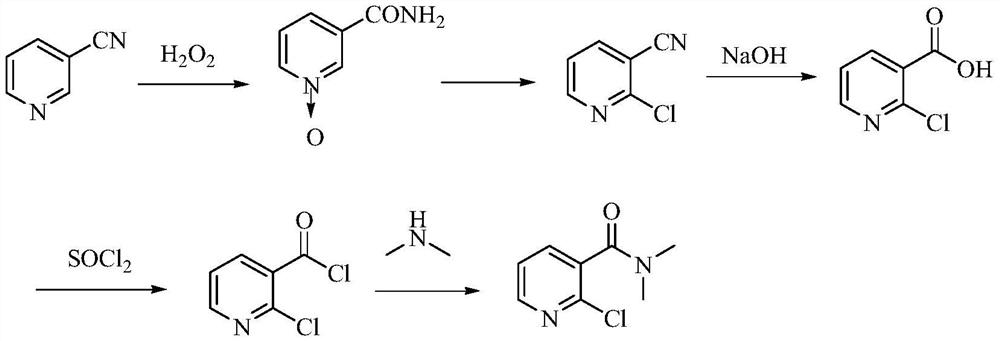
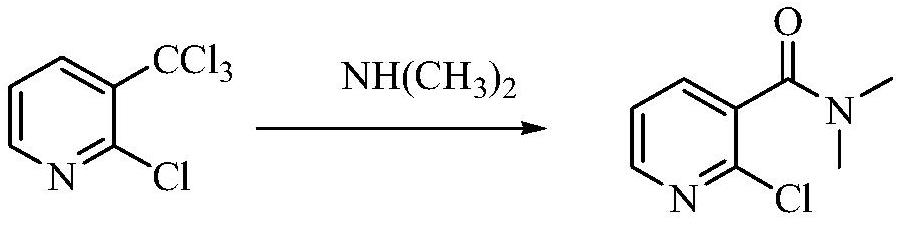
A kind of preparation method of 2-chloro-n,n-dimethylnicotinamide
A technology of dimethyl nicotinamide and alkyl, which is applied in the field of preparation of 2-chloro-N,N-dimethyl nicotinamide, can solve the problems of large amount of waste water, high cost of raw materials, unfavorable industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
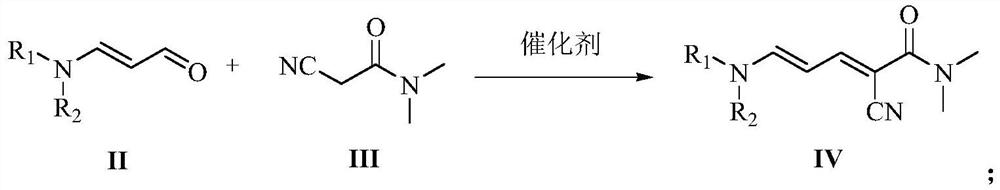
[0042] 1) Synthesis of 2-cyano-5-dimethylamino-N,N-dimethyl-2,4-pentadienamide
[0043]Add 19.8 g (0.2 mol) of 3-dimethylaminoacrolein, 2-cyano-N, N-dimethylethyl Amide (22.4g, 0.2mol) and toluene (180ml), stirred mechanically at room temperature, added acetic acid (2.4g, 0.04mol) and 40% dimethylamine aqueous solution (4.5g, 0.04mol) successively, stirred and heated to reflux at 120°C Water was separated, followed by liquid phase tracking until the reaction was complete, and the reaction was carried out for 11 hours. After the reaction was completed, solvent removal was performed to obtain 38.44 g of brown solid 2-cyano-5-dimethylamino-N,N-dimethyl-2,4-pentadienamide, with a content of 97.5% and a yield of 97.1%.
[0044] 2) Synthesis of 2-chloro-N,N-dimethylnicotinamide
[0045] Add 2-cyano-5-dimethylamino-N,N-dimethyl-2,4-pentadienamide (38.4g, 0.194mol) and dichloroethane (150ml) into a 350ml pressure bottle , stirred and dissolved at 5°C, fed dry hydrogen chloride gas ...
Embodiment 2
[0047] 1) Synthesis of 2-cyano-5-di-n-propylamino-N,N-dimethyl-2,4-pentadienamide
[0048] Add 31.0 g (0.2 mol) of 3-di-n-propylaminoacrolein, 2-cyano-N, N-dimethylethyl Amide (22.4g, 0.2mol) and toluene (180ml), stirred mechanically at room temperature, added acetic acid (2.4g, 0.04mol) and 40% dimethylamine aqueous solution (4.5g, 0.04mol) successively, stirred and heated to reflux at 120°C Water was separated, followed by liquid phase tracking until the reaction was complete, and the reaction was carried out for 14 hours. After the reaction was completed, solvent removal was performed to obtain 49.75 g of brown solid 2-cyano-5-di-n-propylamino-N,N-dimethyl-2,4-pentadienamide, with a content of 97.8% and a yield of 97.7%.
[0049] 2) Synthesis of 2-chloro-N,N-dimethylnicotinamide
[0050] Add 2-cyano-5-di-n-propylamino-N,N-dimethyl-2,4-pentadienamide (49.7g, 0.195mol) and dichloroethane (150ml) into a 350ml pressure bottle , stirred and dissolved at 10°C, fed dry hydrogen...
Embodiment 3
[0052] 1) Synthesis of 2-cyano-5-di-n-propylamino-N,N-dimethyl-2,4-pentadienamide
[0053] Add 31.0 g (0.2 mol) of 3-di-n-propylaminoacrolein, 2-cyano-N, N-dimethylethyl Amide (22.4g, 0.2mol) and toluene (180ml), stirred mechanically at room temperature, added acetic acid (3.6g, 0.06mol) and 40% dimethylamine aqueous solution (6.75g, 0.06mol) successively, stirred and heated to reflux at 120°C Divide the water, follow the liquid phase to detect the complete reaction, and react for 6 hours. After the reaction was completed, solvent removal was performed to obtain 49.85 g of brown solid 2-cyano-5-di-n-propylamino-N,N-dimethyl-2,4-pentadienamide, with a content of 97.5% and a yield of 97.6%.
[0054] 2) Synthesis of 2-chloro-N,N-dimethylnicotinamide
[0055] Add 2-cyano-5-di-n-propylamino-N,N-dimethyl-2,4-pentadienamide (49.8g, 0.195mol) and dichloroethane (150ml) into a 350ml pressure bottle , stirred and dissolved at 10°C, fed dry hydrogen chloride gas until the pressure was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com