Application of placenta multipotential stem cell preparation in preparation of medicine for treatment of acute lung injury
A technology for pluripotent stem cells and acute lung injury, which can be used in drug combinations, medical raw materials derived from mammals, and respiratory diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Preparation of acute lung injury mouse model
[0017] The mice were anesthetized by injecting 250 μl of 1% pentobarbital sodium (prepared with PBS), the skin and muscle layers were cut along the middle of the neck, the trachea was exposed, and a 1 ml syringe was used to puncture the middle of the two rings of the trachea in the centripetal direction, inject 2.5 mg / ml of LPS (prepared in PBS) at a dose of 10 mg / kg body weight. Immediately after the injection, the mice were rotated upright to make the solution evenly distributed on both sides of the lungs. After suture, the mice were fed normally in a sterile environment.
Embodiment 2
[0018] Example 2: Placental pluripotent stem cell preparation
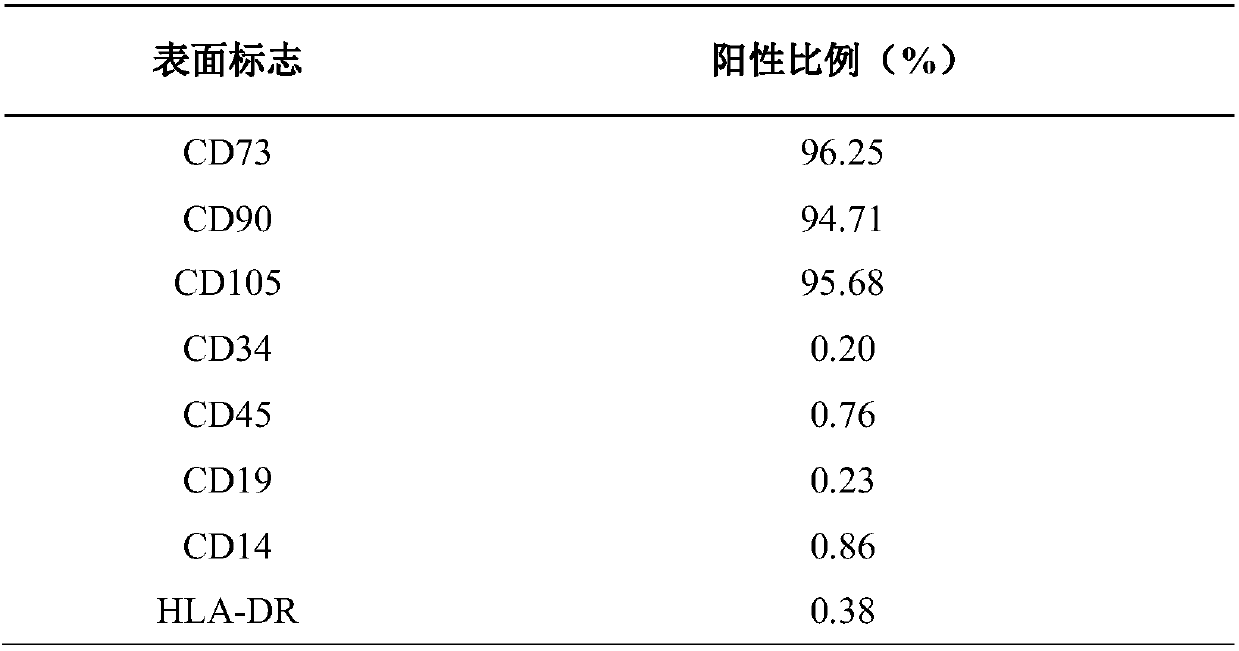
[0019] (1) Isolation, culture and flow cytometric identification of placental pluripotent stem cells
[0020] Isolation and culture of placental pluripotent stem cells: wash the placental surface with physiological saline several times to remove impurities such as congestion and mucus, then quickly disinfect the placental surface with a volume concentration of 75% ethanol solution, and then wash off the residual ethanol solution with physiological saline; From the completed placenta, take the fetal surface tissue into a clean centrifuge tube, and cut it into minced pieces; add 2 times the volume of enzyme solution (type I collagenase with a concentration of 300 IU / mL + DNase I with a concentration of 40 IU / mL, prepared with normal saline) ), digested at 37 °C for 1 hour; the digested product was filtered with a 70 μm filter to remove tissue residues, and a cell suspension was obtained; The isolated mononuclear ce...
Embodiment 3
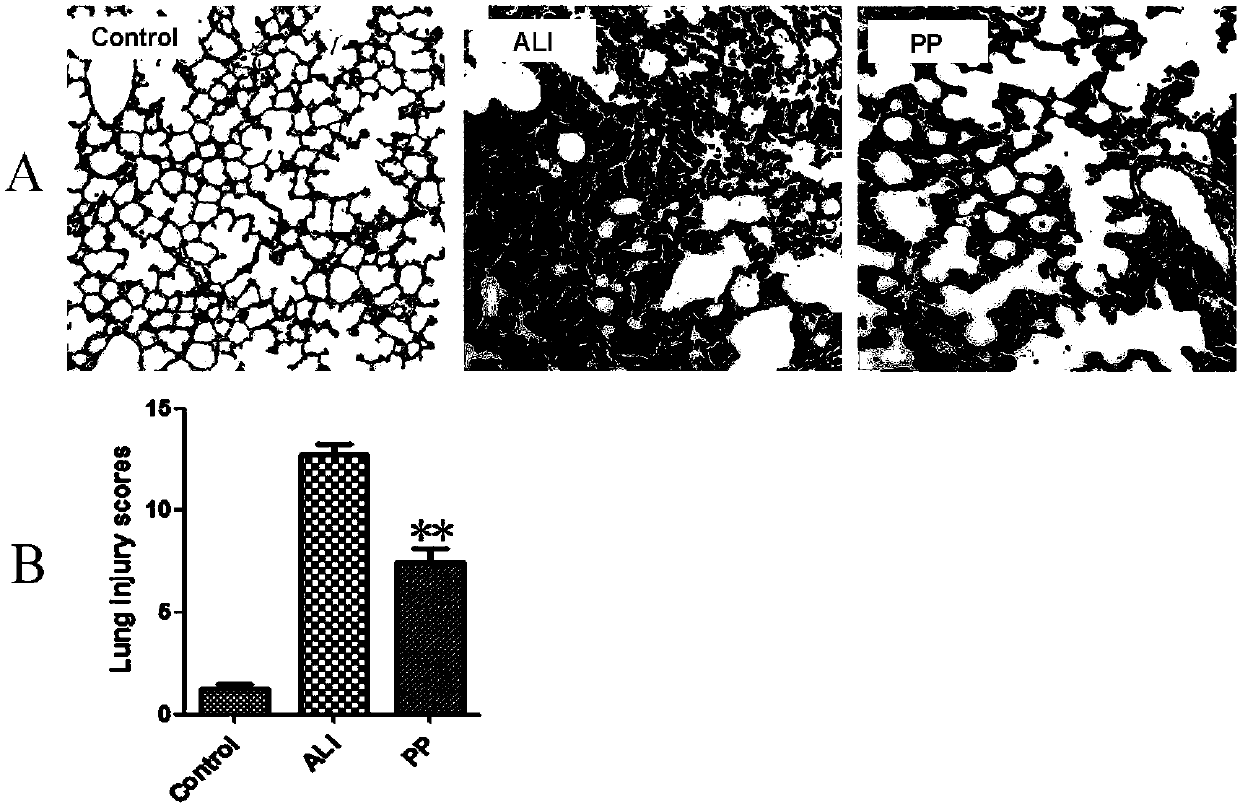
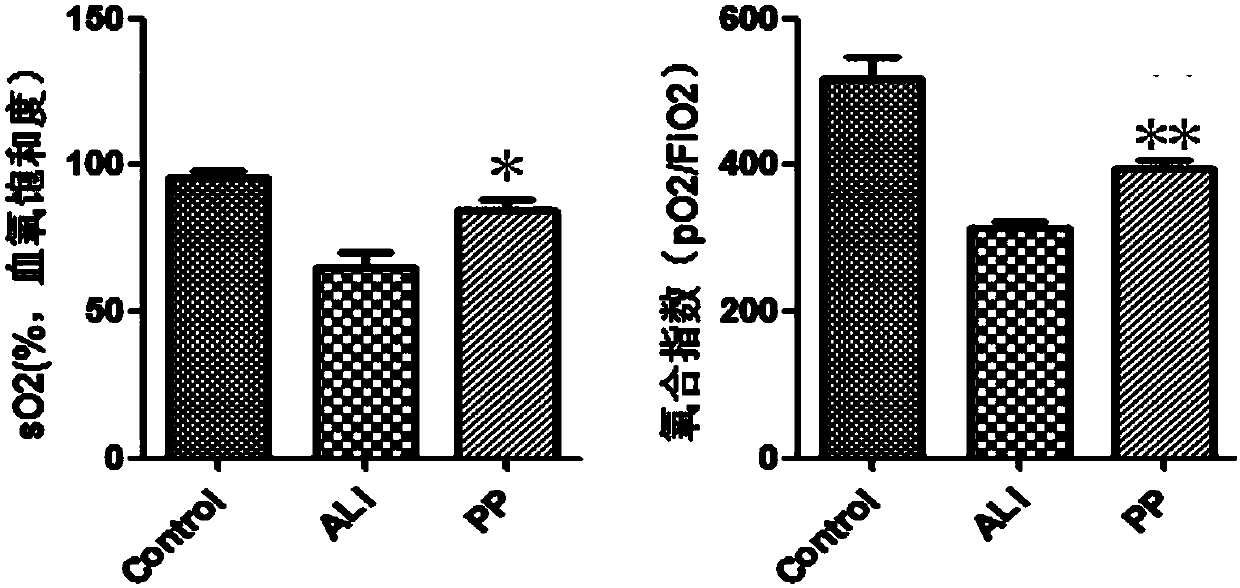
[0026] Example 3: Placental pluripotent stem cell preparation infusion reduces the degree of lung injury and improves lung function
[0027] (1) A mouse acute lung injury (ALI) model established by the method described in Example 1, the experiment was divided into 3 groups: group 1 was the normal group, group 2 was the ALI model group, and group 3 was the ALI model placental pluripotent stem cell preparation injection group. 3 groups 6 hours after modeling, press 8×10 7 The placental pluripotent stem cell preparation prepared in Example 2 was infused through the tail vein at a dose per kg body weight. 90h after injection, samples were taken for lung histopathology and blood gas analysis to analyze the effect of placental pluripotent stem cells on ALI.
[0028] (2) Pathological detection of lung tissue: Take lung tissue, fix it in 4% paraformaldehyde at 4 degrees overnight, then perform paraffin section and HE staining, observe and photograph under microscope, and detect pulm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com