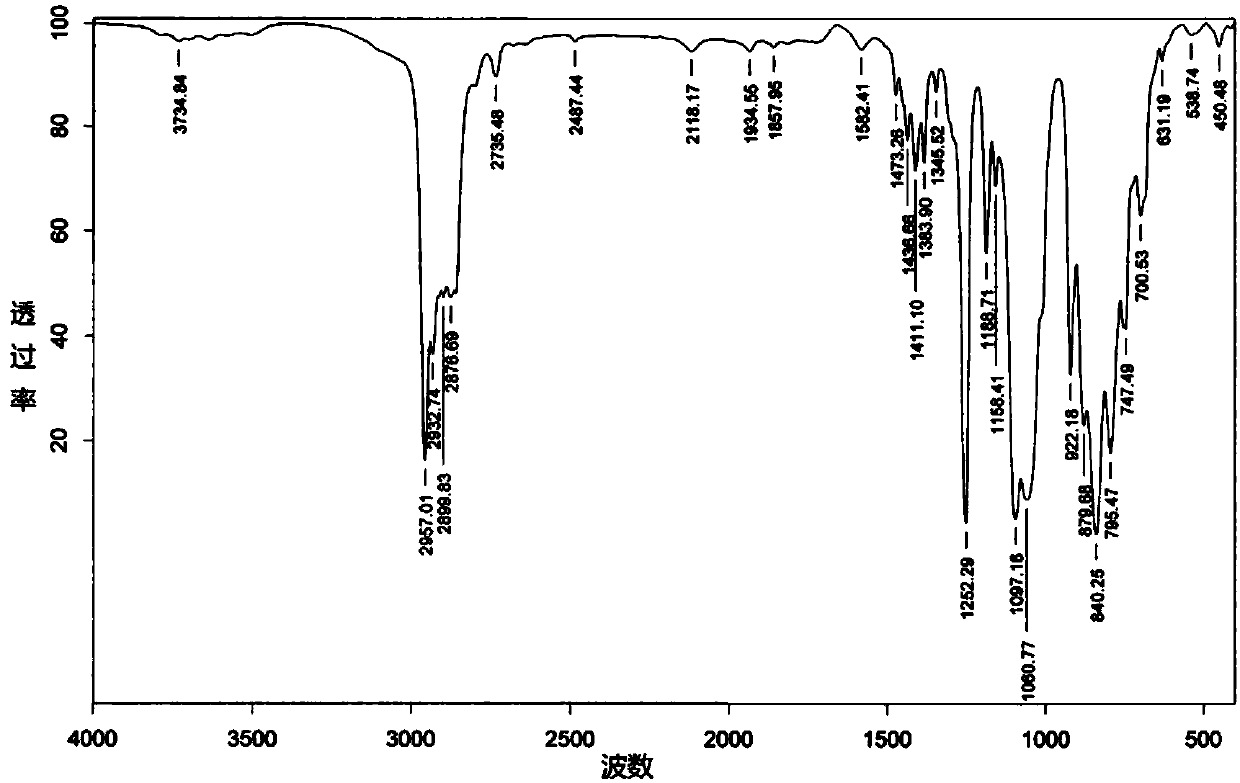
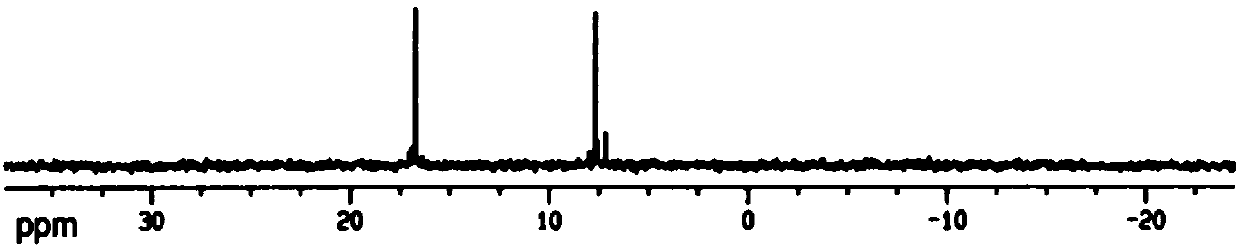
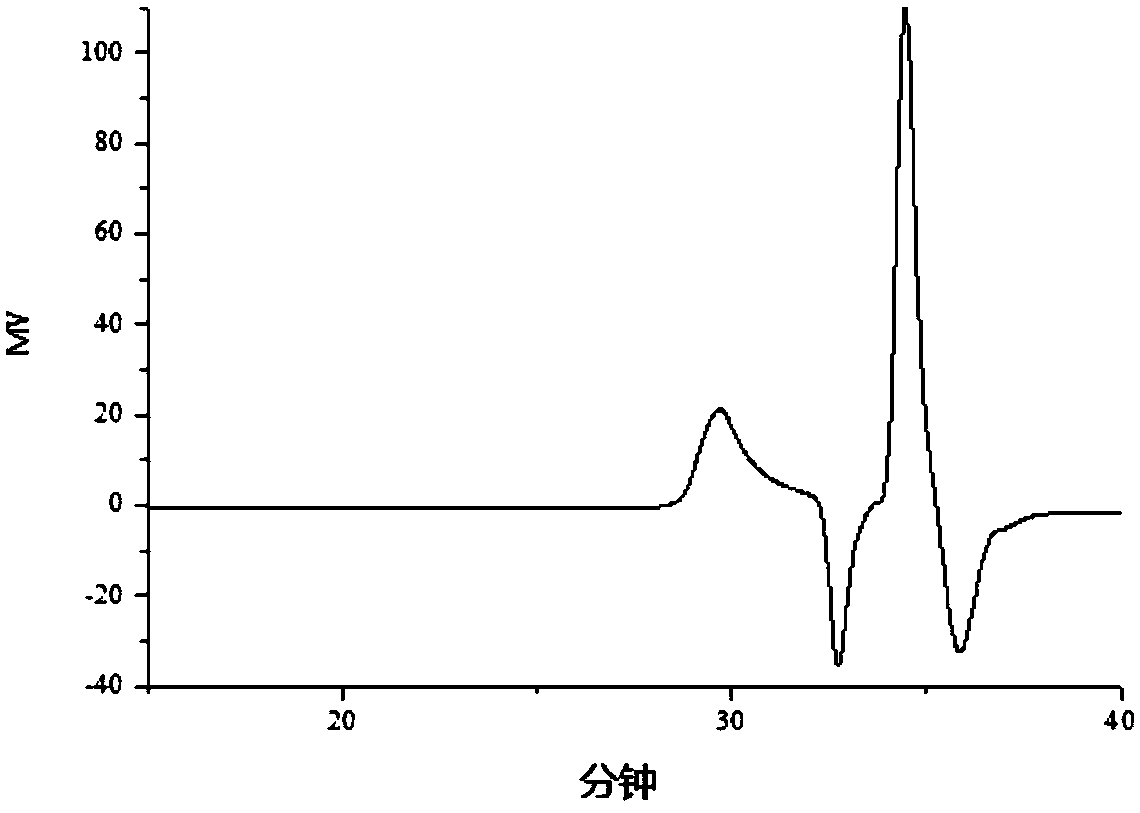
Polycaprolactone modified polysiloxane, preparing method and application
A technology of polysiloxane and polycaprolactone, which is applied in the field of macromolecules, can solve the problems of not explicitly mentioning copolymer examples and not mentioning the preparation method of polycaprolactone-polysiloxane graft copolymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Polycaprolactone-modified polysiloxanes having the formula (I), R 1 for CH 3 CH 2 CH 2 CH 2 , R 2 and R 3 Both CH 2 CH 2 CH 2 , n=1, m=12, x=1.8, y=1.9;
[0137]
[0138] The reaction equation of the polycaprolactone modified polysiloxane with structural formula (I) is as follows:
[0139]
[0140] The first step, hydroxyl protection: In a 1L reactor, slowly add 338.1g (2.1mol) of hexamethyldisilazane dropwise to 232g (4mol) of allyl alcohol A at room temperature 1 Medium (the molar ratio of unsaturated monohydric alcohol to hexamethyldisilazane is 2:1.05). After the dropwise addition, the temperature of the reaction system was raised to 100° C., and the reaction was continued at this temperature for 6 hours, and then the reaction was stopped. The fraction at 98-100° C. was collected under normal pressure to obtain 473.2 g of allyloxytrimethylsilane with a yield of 91%.
[0141] The second step, a hydrosilylation addition: in a 1L reactor, add 393.9g (...
Embodiment 2
[0155] Polycaprolactone-modified polysiloxanes having the formula (II): R 5 for OSi(CH 3 ) 2 O, R 2 , R 3 and R 4 Both CH 2 CH 2 CH 2 CH 2 , (p+q)=1, (o+r)=10, x=2.7, y=2, z=2;
[0156]
[0157] o>0, p>0, q>0, r>0, x>0, y>0, z>0.
[0158] The first step, hydroxyl protection: In a 1L reactor, slowly add 338.1g (2.1mol) hexamethyldisilazane dropwise to 288g 3-buten-1-ol (4mol) A at room temperature 3Medium (the molar ratio of unsaturated monohydric alcohol to hexamethyldisilazane is 2:1.05). After the dropwise addition, the temperature of the reaction system was raised to 100° C., and the reaction was continued at this temperature for 6 hours, and then the reaction was stopped. The fraction at 110-115°C was collected under normal pressure to obtain 558 g of butoxytrimethylsilane with a yield of 96.9%.
[0159] The second step, a hydrosilylation addition: in a 1L reactor, add 436.3g (3mol) enbutoxytrimethylsilane and 0.2g chloroplatinic acid catalyst that are obtai...
Embodiment 3
[0172] Polycaprolactone-modified polysiloxanes having the formula (I), R 1 for CH 3 CH 2 CH 2 CH 2 , R 2 and R 3 Both CH 2 CH 2 CH 2 CH 2 CH 2 , n=2, m=19.6, x=4, y=2;
[0173] The first step, hydroxyl protection: In a 1L reactor, slowly drop 338.1g (2.1mol) hexamethyldisilazane to 344g (4mol) 4-penten-1-ol A at room temperature 4 Medium (the molar ratio of unsaturated monohydric alcohol to hexamethyldisilazane is 2:1.05). After the dropwise addition, the temperature of the reaction system was raised to 100° C., and the reaction was continued at this temperature for 6 hours, and then the reaction was stopped. The fraction at 98-100°C was collected under normal pressure to obtain 600 g of 4-pentenyloxytrimethylsilane with a yield of 94.9%.
[0174] The second step, a hydrosilylation: in the reactor of 1L, add successively the 4-pentenyloxytrimethylsilane and the 0.5g chloroplatinic acid catalyst (the quality of the catalyst) obtained in the first step of 474g (3mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com