Amphiphilic polymeric prodrugs for reducing and releasing original drug and preparation method thereof
An amphiphilic polymer and drug technology, applied in the field of medicine and chemical industry, can solve the problems of inability to distinguish cells, inability to identify tumor sites, etc., and achieve the effects of easy availability of raw materials, mass production, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the synthesis of polyethylene glycol-disulfide bond-SN38
[0048] (1) Preparation of 4-mercaptobutyric acid:
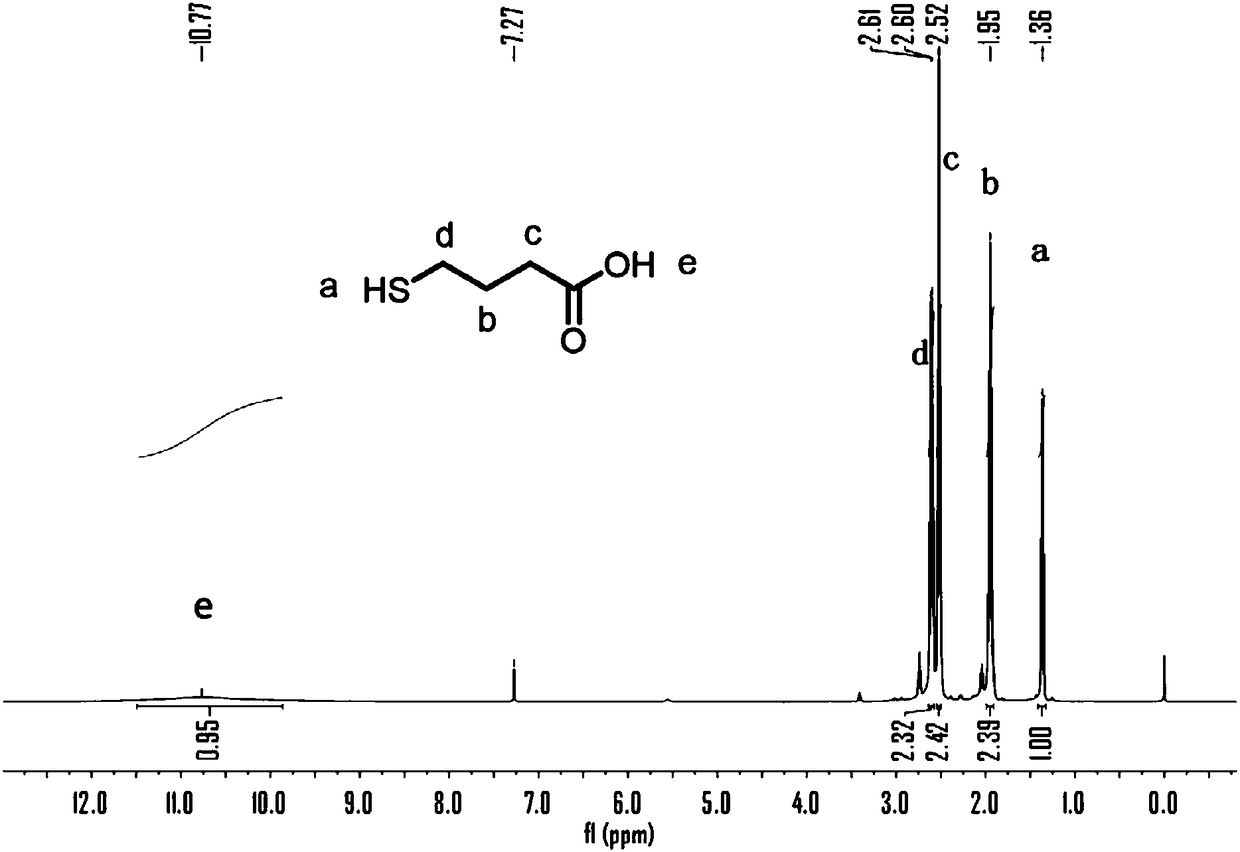
[0049] Add 4-bromobutyric acid (10 g, 59.9 mmol) and thiourea (6.37 g, 83.8 mmol) into 250 mL of ethanol, and reflux for 4 hours. After dissolving NaOH (24 g, 600 mmol) in 250 mL of ethanol, it was added into the reaction system, and the reflux was continued for 16 hours. Cool to room temperature, filter to obtain a white solid, add water to dissolve. The aqueous phase was washed with ether, separated, adjusted to pH 1 with 2M HCl, extracted with ether, dried over anhydrous sodium sulfate, and dried in vacuo to obtain 4.9 g of light yellow oil with a yield of 68%. products tested 1 H NMR shows that the 4-mercaptobutyric acid of structure shown in formula a is obtained, such as figure 1 .
[0050] (2) Preparation of 4-(2-pyridyldithio)butanoic acid:
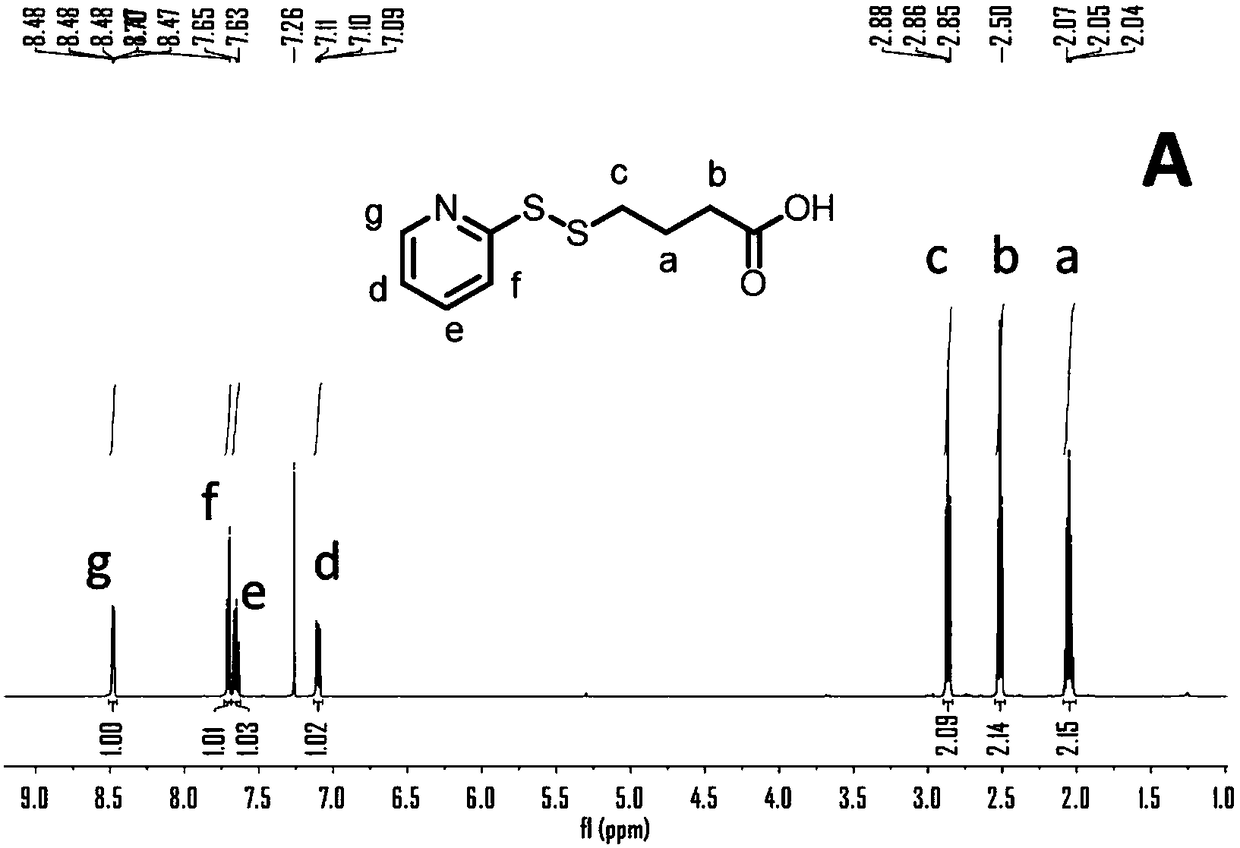
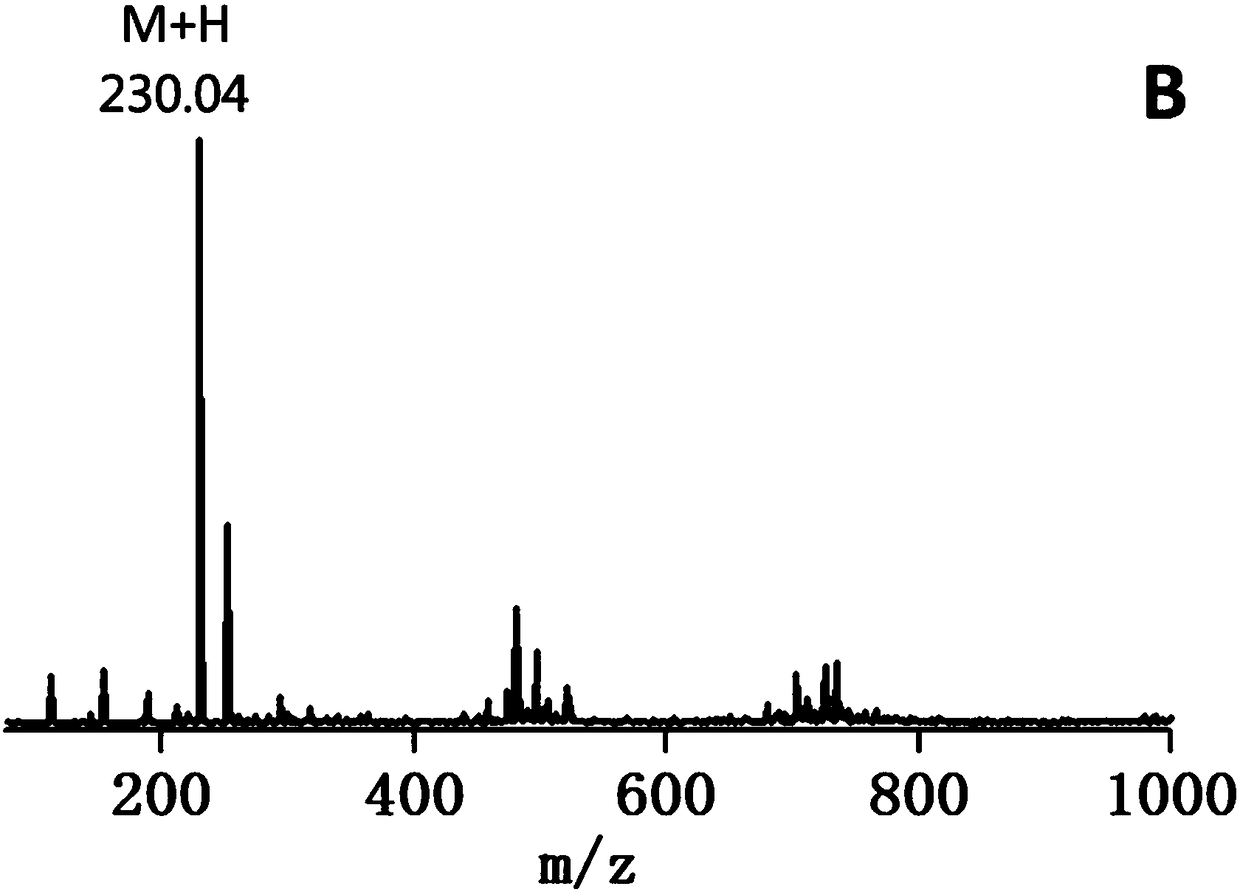
[0051] 4-mercaptobutyric acid (4.9 g, 40.8 mmol) and dithiobipyridine (Py-SS-Py, 18 g, 81.7 ...
Embodiment 2
[0059] Embodiment 2, the synthesis of different molecular weight polyethylene glycol-disulfide bond-SN38
[0060] Synthetic method is the same as embodiment 1, wherein with mPEG 1000 -SH or mPEG 5000 -SH replaces mPEG 2000 -SH, to obtain polyethylene glycol-disulfide bond-SN38 conjugates of different molecular weights.
Embodiment 3
[0061] Embodiment 3, the synthesis of polyethylene glycol-disulfide bond-cabazitaxel
[0062] (1) Preparation of 5-(2-pyridyldithio)pentanoic acid:
[0063] Add 5-bromobutyric acid (2 g, 11 mmol) and thiourea (1.18 g, 15.5 mmol) into 60 mL of ethanol, and reflux for 4 hours. After dissolving NaOH (4.4g, 110mmol) in 60mL of ethanol, it was added into the reaction system, and the reflux was continued for 16 hours. Cool to room temperature, filter to obtain a white solid, add water to dissolve. The aqueous phase was washed with ether, separated, adjusted to pH 1 with 2M HCl, extracted with ether, dried over anhydrous sodium sulfate, and dried in vacuo to obtain 0.88 g of a light yellow oil.
[0064] (2) The light yellow oil and dithiobipyridine (Py-SS-Py, 2.9 g, 13.1 mmol) were dissolved in 30 mL of methanol, and reacted at room temperature for 3 hours. The methanol was distilled off under reduced pressure, separated through a neutral alumina column, concentrated, and dried in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com